Abstract
Background:
Obstructive sleep apnea (OSA) has been shown to be an important risk factor for metabolic syndrome and cardiovascular disease. Endothelial dysfunction plays a pivotal role in the pathophysiology of these diseases. However, little is known about the relationship between sleep apnea and microvascular endothelial dysfunction, as assessed by digital reactive hyperemia.
Methods:
The study population consisted of 80 patients (mean age, 48 ± 12 years-old; 65 men; 59 hypertensive). We measured–apnea hypopnea index (AHI) and mild OSA was defined as 5 < AHI <15 and moderate to severe OSA as AHI ≥ 15. Reactive hyperemia index (RHI) derived from peripheral arterial tonometry (PAT) as measurement of endothelium-mediated vasodilatation.
Results:
There were 61 OSA patients in the study population (AHI, 21.5 ± 16.7 vs. 2.7 ± 1.6 in non-OSA; p < 0.001). There were no significant difference in RHI and peripheral augmentation index (pAIx) between OSA and non-OSA group (RHI, 2.04 ± 0.48 vs. 2.06 ± 0.42; p = 0.894; pAIx, 21.7% ± 24.0% vs. 21.7% ± 30.0%; p = 1.000, respectively). Also, there was no significant difference in RHI and pAIx between mild (n = 31) and moderate to severe (n = 30) OSA group (RHI, 2.10 ± 0.47 vs. 1.98 ± 0.49; p = 0.333; pAIx, 24.2% ± 20.7% vs. 19.0% ± 27.2%; p = 0.407, respectively), either. Overall, no significant correlation between AHI and RHI was observed (r = -0.023, p = 0.837). The other OSA severity indices such as oxygen desaturation index, mean and minimum oxygen saturation were not correlated with RHI or pAIx. In the subgroup analysis for the OSA group, we could not find any significant relationships between AHI and PAT parameters, either.
References
1. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002; 165:1217–39.
2. Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation. 2008; 118:1080–111.
3. Baguet JP, Barone-Rochette G, Tamisier R, Levy P, Pepin JL. Mechanisms of cardiac dysfunction in obstructive sleep apnea. Nat Rev Cardiol. 2012; 9:679–88.

4. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005; 353:2034–41.

5. Gami AS, Pressman G, Caples SM, Kanagala R, Gard JJ, Davison DE, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation. 2004; 110:364–7.

6. Budhiraja R, Parthasarathy S, Quan SF. Endothelial dysfunction in obstructive sleep apnea. J Clin Sleep Med. 2007; 3:409–15.

7. Namtvedt SK, Hisdal J, Randby A, Agewall S, Stranden E, Somers VK, et al. Impaired endothelial function in persons with obstructive sleep apnoea: impact of obesity. Heart. 2013; 99:30–4.

8. Kato M, Roberts-Thomson P, Phillips BG, Haynes WG, Winnicki M, Accurso V, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000; 102:2607–10.

9. Oflaz H, Cuhadaroglu C, Pamukcu B, Meric M, Ece T, Kasikcioglu E, et al. Endothelial function in patients with obstructive sleep apnea syndrome but without hypertension. Respiration. 2006; 73:751–6.

10. Chung S, Yoon IY, Lee CH, Kim JW. The association of nocturnal hypoxemia with arterial stiffness and endothelial dysfunction in male patients with obstructive sleep apnea syndrome. Respiration. 2010; 79:363–9.

11. Chami HA, Keyes MJ, Vita JA, Mitchell GF, Larson MG, Fan S, et al. Brachial artery diameter, blood flow and flow-mediated dilation in sleep-disordered breathing. Vasc Med. 2009; 14:351–60.

12. Kuvin JT, Patel AR, Sliney KA, Pandian NG, Sheffy J, Schnall RP, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003; 146:168–74.

13. Yang WI, Park S, Youn JC, Son NH, Lee SH, Kang SM, et al. Augmentation index association with reactive hyperemia as assessed by peripheral arterial tonometry in hypertension. Am J Hypertens. 2011; 24:1234–8.

14. Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008; 117:2467–74.

15. Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010; 31:1142–8.

16. Hamburg NM, Palmisano J, Larson MG, Sullivan LM, Lehman BT, Vasan RS, et al. Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension. 2011; 57:390–6.
17. Patil SP. What every clinician should know about polysomnography. Respir Care. 2010; 55:1179–95.
19. Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013; 62:569–76.
20. Seif F, Patel SR, Walia H, Rueschman M, Bhatt DL, Gottlieb DJ, et al. Association between obstructive sleep apnea severity and endothelial dysfunction in an increased background of cardiovascular burden. J Sleep Res. 2013; 22:443–51.

21. Heffernan KS, Patvardhan EA, Kapur NK, Karas RH, Kuvin JT. Peripheral augmentation index as a biomarker of vascular aging: an invasive hemodynamics approach. Eur J Appl Physiol. 2012; 112:2871–9.

Fig. 1.
Box diagrams showing (A) RHI and (B) pAIx in patients with no (AHI < 5), mild (5 ≤ AHI < 15) and moderate to severe (AHI ≥ 15) obstructive sleep apnea. In these plots, the upper and lower bars outside the boxes represent the 90% confidence interval. RHI, reactive hyperemia index; pAIx, peripheral augmentation index; AHI, apnea-hyponea index.
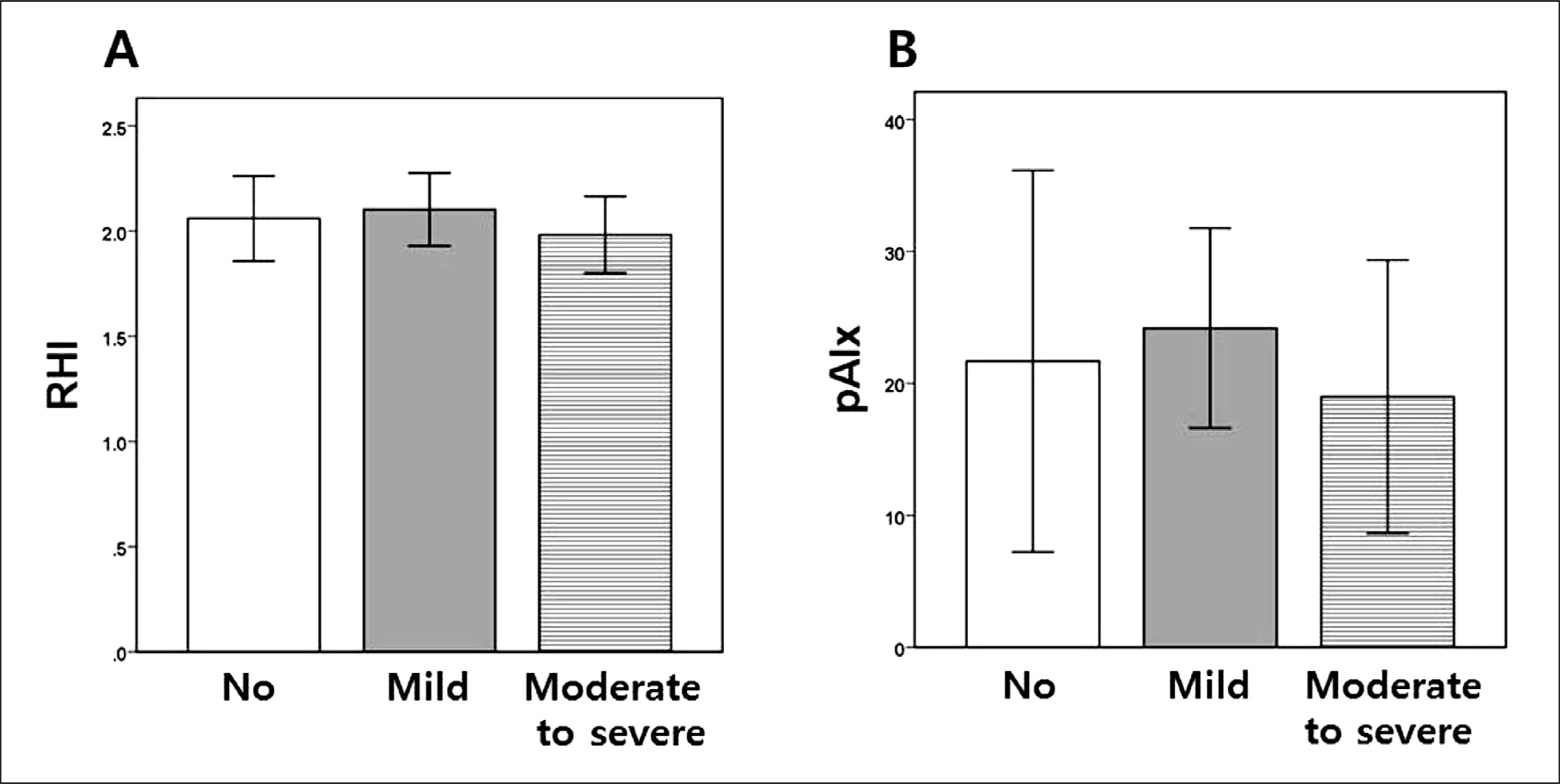
Fig. 2.
Scatter diagrams showing the correlations between AHI and (A) RHI and (B) pAIx. AHI, apnea-hyponea index; RHI, reactive hyperemia index; pAIx, peripheral augmentation index.
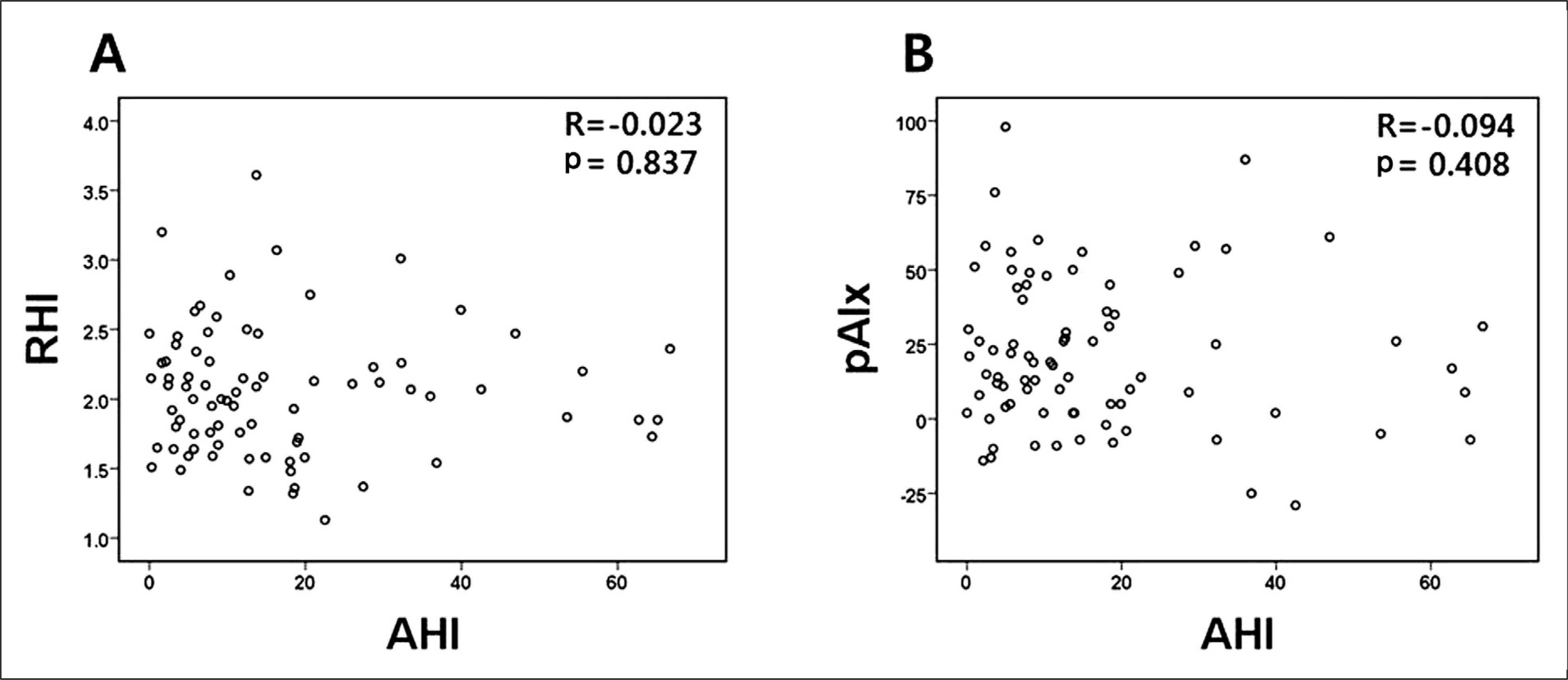
Table 1.
Baseline characteristics of study patients
Table 2.
Correlation analysis with RHI and peripheral AIx in overall groups
Table 3.
Correlation analysis with RHI and peripheral AIx in OSA groups
Table 4.
Multiple regression analysis for the association between reactive hyperemia index and AHI
Table 5.
Multiple regression analysis for the association between peripheral augmentation index and AHI




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download