ABSTRACT
Background:
Endothelial dysfunction has been documented in patients with type 2 diabetes especially when combined with hypertension. We prospectively investigated the effects of pioglitazone in improving endothelial function in hypertensive type 2 diabetic patients during the 6-month follow-up.
Methods:
Hypertensive type 2 diabetic patients were randomly assigned to pioglitazone (n = 25) or placebo (n = 25). Primary endpoint was to compare changes in brachial artery flow-mediated dilation (baFMD) between the 2 groups during the 6-month follow-up. Secondary endpoints were to compare changes in the circulating levels of microRNA-17, -21, 92a, -126, and -145 which have been known as indicators of endothelial cell migration and atherosclerosis progression during the 6-month follow-up. Inflammatory markers such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), high-sensitive C-reactive protein, adiponectin, soluble intercellular adhesion molecule-1 (sICAM-1), and soluble vascular cell adhesion molecule-1 (sVCAM-1) were compared during the follow-up.
Results:
The prevalences of risk factors such as hyperlipidemia, smoking, stroke, and family history of coronary artery disease did not show significant differences between the 2 groups. Increases in baFMD (0.33 ± 0.34 mm vs. 0.02 ± 0.25 mm, p < 0.05, respectively) and in the level of circulating microRNA-21 (0.23 ± 0.05 vs. -0.06 ± 0.04, p < 0.05, respectively) were significantly greater in the pioglitazone group when compared to the placebo group during the 6-month follow-up. No significant differences in the prevalences of new onset heart failure, fracture, and bladder cancer were noted during the follow-up between the 2 groups. Decreases in the levels of inflammatory marker such as IL-6 (-2.54 ± 2.32 pg/mL vs. -1.34 ± 2.12 pg/mL, p < 0.05, respectively), TNF-α (-1.54 ± 1.51 pg/mL vs. 0.14 ± 1.12 pg/mL, p < 0.05, respectively), sICAM-1 (-39 ± 52 ng/mL vs. 6 ± 72 ng/mL, p < 0.05, respectively), and sVCAM-1 (-154 ± 198 ng/mL vs. -11 ± 356 ng/mL, p < 0.05, respectively) were significantly greater in the pioglitazone group compared to the placebo group during the follow-up.
References
1. Moody WE, Edwards NC, Madhani M, Chue CD, Steeds RP, Ferro CJ. et al. Endothelial dysfunction and cardiovascular disease in early-stage chronic kidney disease: cause or association? Atherosclerosis. 2012; 223:86–94.
2. Weiss TW, Arnesen H, Seljeflot I. Components of the interleukin-6 transsignalling system are associated with the metabolic syndrome, endothelial dysfunction and arterial stiffness. Metabolism. 2013; 62:1008–13.

3. Hong SJ, Ahn TH, Baek SH, Cho WH, Jeon HK, Kwan J, et al. Comparison of efficacy and tolerability of amlodipine orotate versus amlodipine besylate in adult patients with mild to moderate hypertension: a multicenter, randomized, double-blind, placebo-controlled, parallel-group, 8-week follow-up, noninferiority trial. Clin Ther. 2006; 28:537–51.

4. Bakris G, Briasoulis A, Dahlof B, Jamerson K, Weber MA, Kelly RY, et al. Comparison of benazepril plus amlodipine or hydrochlorothiazide in high-risk patients with hypertension and coronary artery disease. Am J Cardiol. 2013; 112:255–9.

5. Lee DC, Sui X, Church TS, Lavie CJ, Jackson AS, Blair SN. Changes in fitness and fatness on the development of cardiovascular disease risk factors hypertension, metabolic syndrome, and hypercholesterolemia. J Am Coll Cardiol. 2012; 59:665–72.
6. Hong SJ, Choi SC, Ahn CM, Park JH, Kim JS, Lim DS. Telmisartan reduces neointima volume and pulse wave velocity 8 months after zotarolimus-eluting stent implantation in hypertensive type 2 diabetic patients. Heart. 2011; 97:1425–32.

7. Hong SJ, Kim ST, Kim TJ, Kim EO, Ahn CM, Park JH, et al. Cellular and molecular changes associated with inhibitory effect of pioglitazone on neointimal growth in patients with type 2 diabetes after zotarolimus-eluting stent implantation. Arterioscler Thromb Vasc Biol. 2010; 30:2655–65.

8. Jin DK, Lee SJ. How much do we lower the blood pressure in hypertensive patients with diabetes? J Korean Soc Hypertens. 2011; 17:10–6.
9. Mizoguchi M, Tahara N, Tahara A, Nitta Y, Kodama N, Oba T, et al. Pioglitazone attenuates atherosclerotic plaque inflammation in patients with impaired glucose tolerance or diabetes a prospective, randomized, comparator-controlled study using serial FDG PET/CT imaging study of carotid artery and ascending aorta. JACC Cardiovasc Imaging. 2011; 4:1110–8.
10. Hsiao FY, Hsieh PH, Huang WF, Tsai YW, Gau CS. Risk of bladder cancer in diabetic patients treated with rosiglitazone or pioglitazone: a nested case-control study. Drug Saf. 2013; 36:643–9.

11. Ferwana M, Firwana B, Hasan R, Al-Mallah MH, Kim S, Montori VM, et al. Pioglitazone and risk of bladder cancer: a meta-analysis of controlled studies. Diabet Med. 2013; 30:1026–32.

12. Aubert RE, Herrera V, Chen W, Haffner SM, Pendergrass M. Rosiglitazone and pioglitazone increase fracture risk in women and men with type 2 diabetes. Diabetes Obes Metab. 2010; 12:716–21.

13. Loke YK, Singh S, Furberg CD. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ. 2009; 180:32–9.

14. Fichtlscherer S, Zeiher AM, Dimmeler S. Circulating microRNAs: biomarkers or mediators of cardiovascular diseases? Arterioscler Thromb Vasc Biol. 2011; 31:2383–90.
15. Meigs JB, Hu FB, Rifai N, Manson JE. Biomarkers of endothelial dysfunction and risk of type 2 diabetes mellitus. JAMA. 2004; 291:1978–86.

16. Libby P, Plutzky J. Inflammation in diabetes mellitus: role of peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma agonists. Am J Cardiol. 2007; 99:27B–40B.
17. Hallberg C, Rosengren B, Camejo G. Effect of fatty acid on HDL-mediated efflux of cholesterol in THP-1 uman macrophages and human monocytes-derived macrophages. Chem Phys Lipids. 2005; 136:132–3.
18. Jin RC, Min PK, Chan SY. MicroRNA in the diseased pulmonary vasculature: implications for the basic scientist and clinician. J Korean Soc Hypertens. 2013; 19:1–16.

19. Jazbutyte V, Thum T. MicroRNA-21: from cancer to cardiovascular disease. Current Drug Targets. 2010; 11:926–35.

20. Cheng Y, Zhang C. MicroRNA-21 in cardiovascular disease. J Cardiovasc Transl Res. 2010; 3:251–5.

21. Kaluza D, Kroll J, Gesierich S, Manavski Y, Boeckel JN, Doebele C, et al. Histone deacetylase 9 promotes angiogenesis by targeting the antiangiogenic microRNA-17-92 cluster in endothelial cells. Arterioscler Thromb Vasc Biol. 2013; 33:533–43.

22. Wu W, Xiao H, Laguna-Fernandez A, Villarreal G. Jr., Wang KC, Geary GG, et al. Flow-Dependent Regulation of Kruppel-Like Factor 2 Is Mediated by MicroRNA-92a. Circulation. 2011; 124:633–41.
23. Wei Y, Nazari-Jahantigh M, Neth P, Weber C, Schober A. MicroRNA-126, -145. and -155: a therapeutic triad in athero-sclerosis? Arterioscler Thromb Vasc Biol. 2013; 33:449–54.
24. Maachi M, Pieroni L, Bruckert E, Jardel C, Fellahi S, Hainque B, et al. Systemic low-grade inflammation is related to both circulating and adipose tissue TNF alpha, leptin and IL-6 levels in obese women. Int J Obesity. 2004; 28:993–7.
25. You SH, Kim BS, Hong SJ, Ahn CM, Lim DS. The effects of pioglitazone in reducing atherosclerosis progression and neo-intima volume in type 2 diabetic patients: prospective randomized study with volumetric intravascular ultrasonography analysis. Korean Circ J. 2010; 40:625–31.

26. Hong SJ, Park CG, Seo HS, Kim JW, Rha SW, Oh DJ. Decrease in plasma adiponectin concentrations in patients with variant angina and acute coronary syndrome. Am J Cardiol. 2005; 95:61a–2a.
Fig. 1.
Comparison of changes in brachial artery flow-mediated dilatation during the 6-month follow-up between the pioglitazone and control groups.
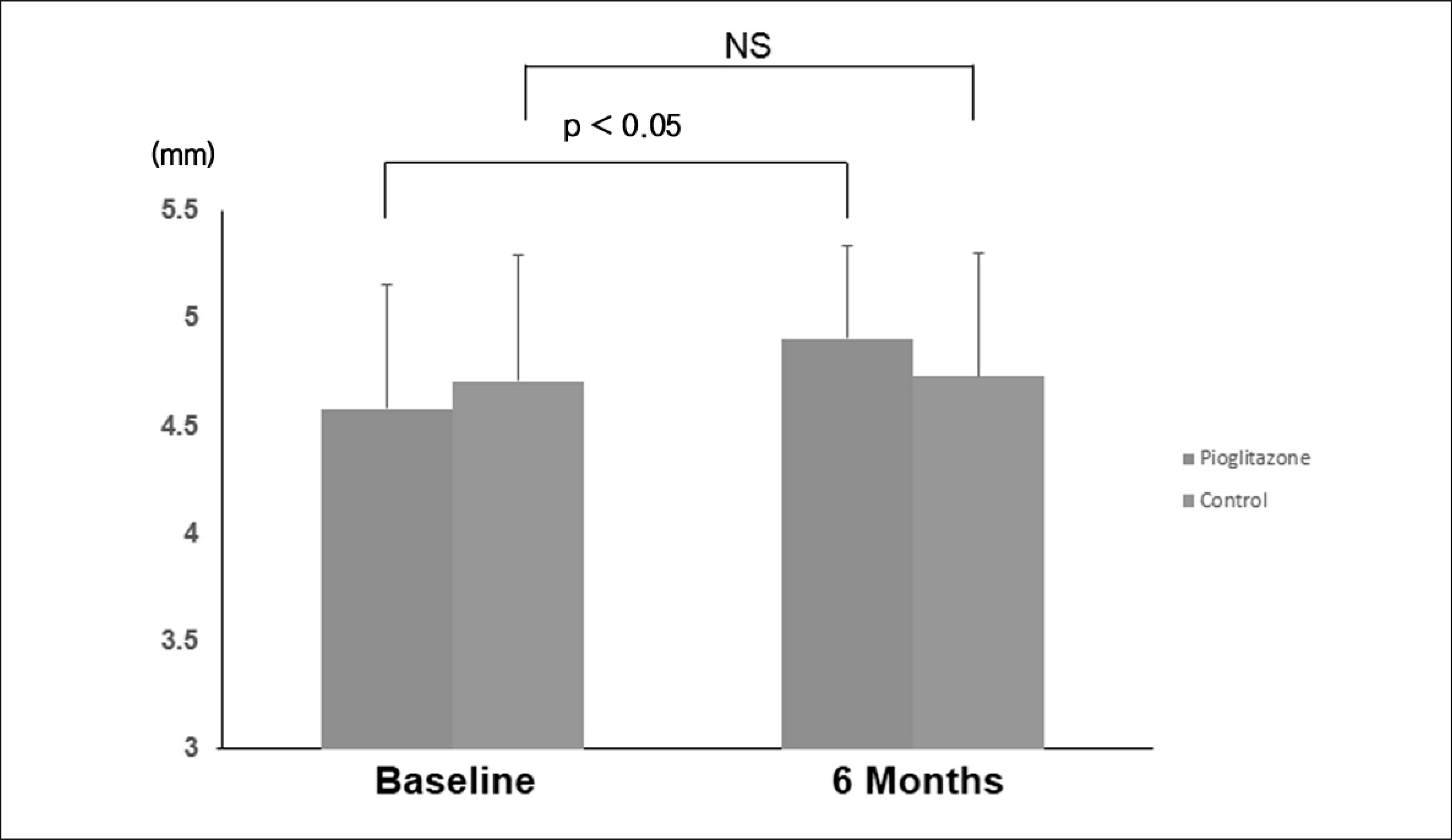
Fig. 2.
(A) MicroRNA-21 increased significantly only in the pioglitazone group during the 6-month F/U, and (B) significant correlation was found between the changes in microRNA-21 and changes in brachial artery flow-mediated dilatation. (C) No significant differences were found with changes in microRNA-17, -92a, -126, and -145. baFMD, brachial artery flow-mediated dilation; F/U, follow-up.
Table 1.
Baseline patient characteristics
Table 2.
Changes in brachial artery flow-mediated dilatation during the 6-month follow-up
| Variable | Pioglitazone group (n = 25) | Control group (n = 25) | ||
|---|---|---|---|---|
| Baseline | After 6 months | Baseline | After 6 months | |
| Brachial artery diameter at rest (mm) | 4.47 ± 0.58 | 4.51 ± 0.49 | 4.59 ± 0.59 | 4.57 ± 0.57 |
| Flow-mediated dilatation (mm) | 4.58 ± 0.58 | 4.91 ± 0.43*,† | 4.71 ± 0.59 | 4.73 ± 0.58 |
| Changes from at rest (mm) | 0.11 ± 0.06 | 0.40 ± 0.08*,† | 0.12 ± 0.07 | 0.16 ± 0.07 |
| Changes from baseline (mm) | 0.33 ± 0.34† | 0.02±0.25 | ||
Table 3.
Changes in the level of inflammatory markers during the 6-month follow-up
| Variable | Pioglitazone group (n = 25) | Control group (n = 25) | ||
|---|---|---|---|---|
| Baseline | After 6 months | Baseline | After 6 months | |
| Interleukin-6 (pg/mL) | 3.91 ± 3.12 | 1.45 ± 1.10* | 3.56 ± 3.21 | 2.23 ± 1.71* |
| Changes from baseline (pg/mL) | -2.54 ± 2.32† | -1.34 ± 2.12 | ||
| Tumor necrosis factor-α (pg/mL) | 5.71 ± 4.19 | 4.16 ± 2.56* | 5.16 ± 3.41 | 4.99 ± 3.11 |
| Changes from baseline (pg/mL) | -1.54 ± 1.51† | 0.14 ± 1.12 | ||
| High-sensitive C-reactive protein (mg/L) | 4.89 ± 4.11 | 1.80 ± 1.57* | 5.11 ± 4.22 | 1.89 ± 1.81* |
| Changes from baseline (mg/L) | -3.09 ± 3.01 | -3.21 ± 2.98 | ||
| Adiponectin (μ g/mL) | 5.29 ± 4.17 | 6.76 ± 4.14* | 5.55 ± 3.85 | 6.49 ± 4.66* |
| Changes from baseline (μ g/mL) | 1.47 ± 0.89† | 0.98 ± 0.88 | ||
| sICAM-1 (ng/mL) | 430 ± 154 | 391 ± 133* | 416 ± 131 | 422 ± 172 |
| Changes from baseline (ng/mL) | -39 ± 52† | 6 ± 72 | ||
| sVCAM-1 (ng/mL) | 1107 ± 344 | 954 ± 328* | 1088 ± 429 | 1077 ± 401 |
| Changes from baseline (ng/mL) | -154 ± 198† | -11 ± 356 | ||
Table 4.
Changes in the level of lipid profiles during the 6-month follow-up
| Variable | Pioglitazone group (n = 25) | Control group (n = 25) | ||
|---|---|---|---|---|
| Baseline | After 6 months | Baseline | After 6 months | |
| Total cholesterol (mg/dL) | 220 ± 54 | 144 ± 41* | 215 ± 44 | 141 ± 32* |
| Changes from baseline (mg/dL) | -76 ± 62 | -75 ± 43 | ||
| Low density lipoprotein cholesterol (mg/dL) | 143 ± 73 | 73 ± 53* | 146 ± 108 | 77 ± 39* |
| Changes from baseline (mg/dL) | -70 ± 39 | -68 ± 42 | ||
| High density lipoprotein cholesterol (mg/dL) | 44 ± 27 | 46 ± 18 | 40 ± 22 | 45 ± 12 |
| Changes from baseline (mg/dL) | 2 ± 9 | 4 ± 7 | ||
| Triglyceride (mg/dL) | 127 ± 71 | 110 ± 94 | 123 ± 74 | 113 ± 69 |
| Changes from baseline (mg/dL) | -16 ± 70 | -10 ± 59 | ||
Table 5.
Comparison of adverse clinical events between the 2 groups during the 6-month follow-up




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download