ABSTRACT
Since the first descriptions of their active functions more than ten years ago, small non-coding RNA species termed microRNA (miRNA) have emerged as essential regulators in a broad range of adaptive and maladaptive cellular processes. With an exceptionally rapid pace of discovery in this field, the dysregulation of many individual miRNAs has been implicated in the development and progression of various cardiovascular diseases. MiRNA are also expected to play crucial regulatory roles in the progression of pulmonary vascular diseases such as pulmonary hypertension (PH), yet direct insights in this field are only just emerging. This review will provide an overview of pulmonary hypertension and its molecular mechanisms, tailored for both basic scientists studying pulmonary vascular biology and physicians who manage PH in their clinical practice. We will describe the pathobiology of pulmonary hypertension and mechanisms of action of miRNA relevant to this disease. Moreover, we will summarize the potential roles of miRNA as biomarkers and therapeutic targets as well as future strategies for defining the cooperative actions of these powerful effectors in pulmonary vascular disease.
References
1. Alexander RP, Fang G, Rozowsky J, Snyder M, Gerstein MB. Annotating non-coding regions of the genome. Nat Rev Genet. 2010; 11:559–71.

2. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009; 10:155–9.

4. Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005; 120:15–20.

5. Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009; 19:92–105.

6. Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001; 293:1146–50.

8. Brodersen P, Voinnet O. Revisiting the principles of microRNA target recognition and mode of action. Nat Rev Mol Cell Biol. 2009; 10:141–8.

9. Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003; 115:787–98.

11. Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008; 9:102–14.

13. Liu N, Olson EN. MicroRNA regulatory networks in cardiovascular development. Dev Cell. 2010; 18:510–25.

14. Small EM, Olson EN. Pervasive roles of microRNAs in cardiovascular biology. Nature. 2011; 469:336–42.

15. Abdellatif M. Differential expression of microRNAs in different disease states. Circ Res. 2012; 110:638–50.

16. Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008; 79:581–8.

17. White K, Loscalzo J, Chan SY. Holding our breath: the emerging and anticipated roles of microRNA in pulmonary hypertension. Pulm Circ. 2012; 2:278–90.

18. McLaughlin VV, Davis M, Cornwell W. Pulmonary arterial hypertension. Curr Probl Cardiol. 2011; 36:461–517.

19. Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol. 2011; 8:443–55.

20. Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009; 54(1 Suppl):S43–54.

21. Lourenco AP, Fontoura D, Henriques-Coelho T, Leite-Moreira AF. Current pathophysiological concepts and management of pulmonary hypertension. Int J Cardiol. 2012; 155:350–61.
22. Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol. 2009; 54(1 Suppl):S20–31.

23. Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: new concepts and experimental therapies. Circulation. 2010; 121:2045–66.
24. Tuder RM. Pathology of pulmonary arterial hypertension. Semin Respir Crit Care Med. 2009; 30:376–85.

25. Jeffery TK, Morrell NW. Molecular and cellular basis of pulmonary vascular remodeling in pulmonary hypertension. Prog Cardiovasc Dis. 2002; 45:173–202.

26. Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation. 2004; 109:159–65.

27. Frid MG, Brunetti JA, Burke DL, Carpenter TC, Davie NJ.
Reeves JT, et al. Hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage. Am J Pathol. 2006. 168:659–69.
28. Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005; 96:442–50.
29. International PPH Consortium , Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA 3rd, et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000. 26:p. 81–4.
30. Newman JH, Wheeler L, Lane KB, Loyd E, Gaddipati R, Phillips JA 3rd, et al. Mutation in the gene for bone morphogenetic protein receptor II as a cause of primary pulmonary hypertension in a large kindred. N Engl J Med. 2001; 345:319–24.

31. Eddahibi S, Chaouat A, Morrell N, Fadel E, Fuhrman C, Bugnet AS, et al. Polymorphism of the serotonin transporter gene and pulmonary hypertension in chronic obstructive pulmonary disease. Circulation. 2003; 108:1839–44.

32. Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, et al. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010; 121:2661–71.
34. Chan SY, Loscalzo J. Pathogenic mechanisms of pulmonary arterial hypertension. J Mol Cell Cardiol. 2008; 44:14–30.

35. Chan SY, Loscalzo J. MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle. 2010; 9:1072–83.

36. Wang V, Davis DA, Haque M, Huang LE, Yarchoan R. Differential gene up-regulation by hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha in HEK293T cells. Cancer Res. 2005; 65:3299–306.
37. Maynard MA, Evans AJ, Hosomi T, Hara S, Jewett MA, Ohh M, Human HIF-. 3alpha4 is a dominant-negative regulator of HIF-1 and is down-regulated in renal cell carcinoma. FASEB J. 2005; 19:1396–406.
38. Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008; 15:621–7.
39. Adams JM, Difazio LT, Rolandelli RH, Lujan JJ, Hasko G, Csoka B, et al. HIF-1: a key mediator in hypoxia. Acta Physiol Hung. 2009; 96:19–28.
40. Shimoda LA. Semenza GL. HIF and the lung: role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med. 2011; 183:152–6.
41. Parikh VN, Jin RC, Rabello S, Gulbahce N, White K, Hale A, et al. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation. 2012; 125:1520–32.
42. Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, et al. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol. 2010; 30:716–23.

43. Yang S, Banerjee S. Freitas Ad, Cui H, Xie N, Abraham E, et al. miR-21 regulates chronic hypoxia-induced pulmonary vascular remodeling. Am J Physiol Lung Cell Mol Physiol. 2012; 302:L521–9.
44. Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med. 2011; 208:535–48.

45. Bockmeyer CL, Maegel L, Janciauskiene S, Rische J, Lehmann U, Maus UA, et al. Plexiform vasculopathy of severe pulmonary arterial hypertension and microRNA expression. J Heart Lung Transplant. 2012; 31:764–72.

46. Brock M, Trenkmann M, Gay RE, Michel BA, Gay S, Fischler M, et al. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ Res. 2009; 104:1184–91.

47. Pullamsetti SS, Doebele C, Fischer A, Savai R, Kojonazarov B, Dahal BK, et al. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am J Respir Crit Care Med. 2012; 185:409–19.

48. Brock M, Samillan VJ, Trenkmann M, Schwarzwald C, Ulrich S, Gay RE, et al. AntagomiR directed against miR-20a restores functional BMPR2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. Eur Heart J. 2012; Mar 26 [Epub].

49. Caruso P, Dempsie Y, Stevens HC, McDonald RA, Long L, Lu R, et al. A role for miR-145 in pulmonary arterial hypertension: evidence from mouse models and patient samples. Circ Res. 2012; 111:290–300.
50. Guo L, Qiu Z, Wei L, Yu X, Gao X, Jiang S, et al. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-α1C. Hypertension. 2012; 59:1006–13.

51. Kim J, Kang Y, Kojima Y, Lighthouse JK, Hu X, Aldred MA, et al. An endothelial apelin-FGF link mediated by miR-424 and miR-503 is disrupted in pulmonary arterial hypertension. Nat Med. 2013; 19:74–82.

52. Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008; 105:10513–8.

53. Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol. 2008; 141:672–5.

54. Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008; 18:997–1006.

55. Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009; 2:ra81.

56. Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007; 9:654–9.

57. Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011; 108:5003–8.

58. Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011; 13:423–33.

59. Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M, et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet. 2011; 4:446–54.

60. Rhodes CJ, Wharton J, Boon RA, Roexe T, Tsang H, Wojciak-Stothard B, et al. Reduced microRNA-150 is associated with poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013; 187:294–302.

61. Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010; 39:133–44.

62. Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, et al. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010; 107:6328–33.

63. Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011; 2:282.

64. Fichtlscherer S, Zeiher AM, Dimmeler S. Circulating microRNAs: biomarkers or mediators of cardiovascular diseases? Arterioscler Thromb Vasc Biol. 2011; 31:2383–90.
65. Xu J, Zhao J, Evan G, Xiao C, Cheng Y, Xiao J. Circulating microRNAs: novel biomarkers for cardiovascular diseases. J Mol Med (Berl). 2012; 90:865–75.

66. Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012; 110:483–95.
67. Fabani MM, Abreu-Goodger C, Williams D, Lyons PA, Torres AG, Smith KG, et al. Efficient inhibition of miR-155 function in vivo by peptide nucleic acids. Nucleic Acids Res. 2010; 38:4466–75.

68. Lennox KA, Behlke MA. Chemical modification and design of anti-miRNA oligonucleotides. Gene Ther. 2011; 18:1111–20.

69. Montgomery RL. van Rooij E. Therapeutic advances in MicroRNA targeting. J Cardiovasc Pharmacol. 2011; 57:1–7.
70. Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature. 2005; 438:685–9.

71. Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, et al. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007; 13:613–8.

72. Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008; 456:980–4.

73. Ma L, Reinhardt F, Pan E, Soutschek J, Bhat B, Marcusson EG, et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol. 2010; 28:341–7.

74. Babar IA, Cheng CJ, Booth CJ, Liang X, Weidhaas JB.
Saltzman WM, et al. Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc Natl Acad Sci U S A. 2012. 109:E1695–704.
Fig. 1.
MicroRNA (miRNA) biogenesis and mechanism of action. MiRNAs undergo several nuclear and cytosolic processing steps before maturation to a biologically active form (19-24 nt). After processing, the mature miRNA is incorporated into the RNA?induced silencing complex (RISC). Through binding to the complementary sites in the 3’ untranslated region of their target genes, miRNA promote down? regulation of the protein synthesis via translational repression or messenger RNA (mRNA) degradation.
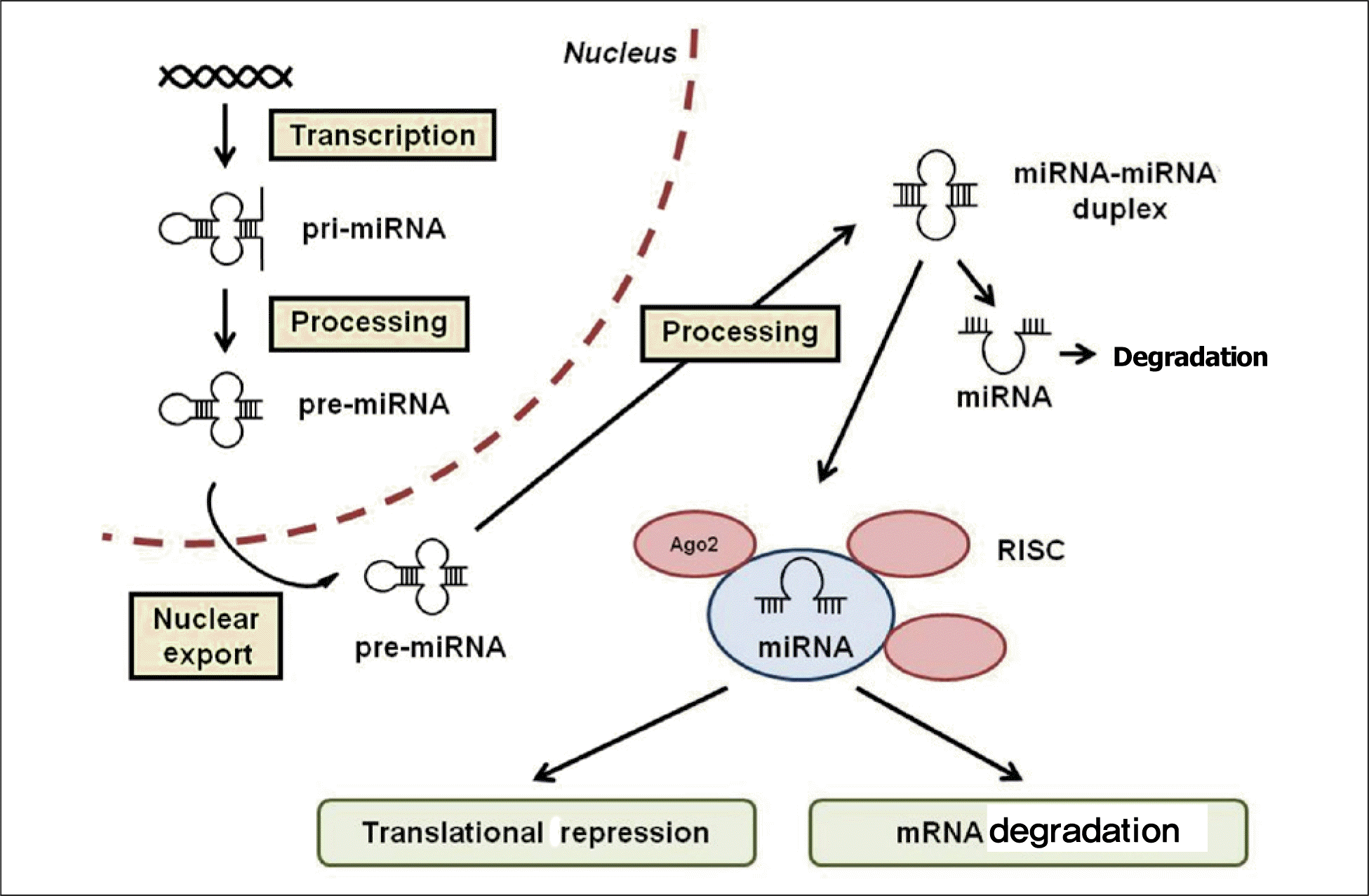
Fig. 2.
Role of microRNAs (miRNAs) in cardiovascular disease. (A) Control of cardiomyocyte function by miRNA. The pathogenic events influencing cardiomyocytes in ischemic and hypertrophic diseases are closely controlled by multiple miRNA which regulate cell survival, apoptosis, angiogenesis, fibrosis, and remodeling.14) (B) Control of vascular function by miRNA. Pathophenotypes in diseased vasculature are closely controlled by miRNA, which regulate vascular integrity and remodeling.15,16) From White K, et al. Pulm Circ. 2012;2:278-90, with permission of Pulmonary Circulation.17)
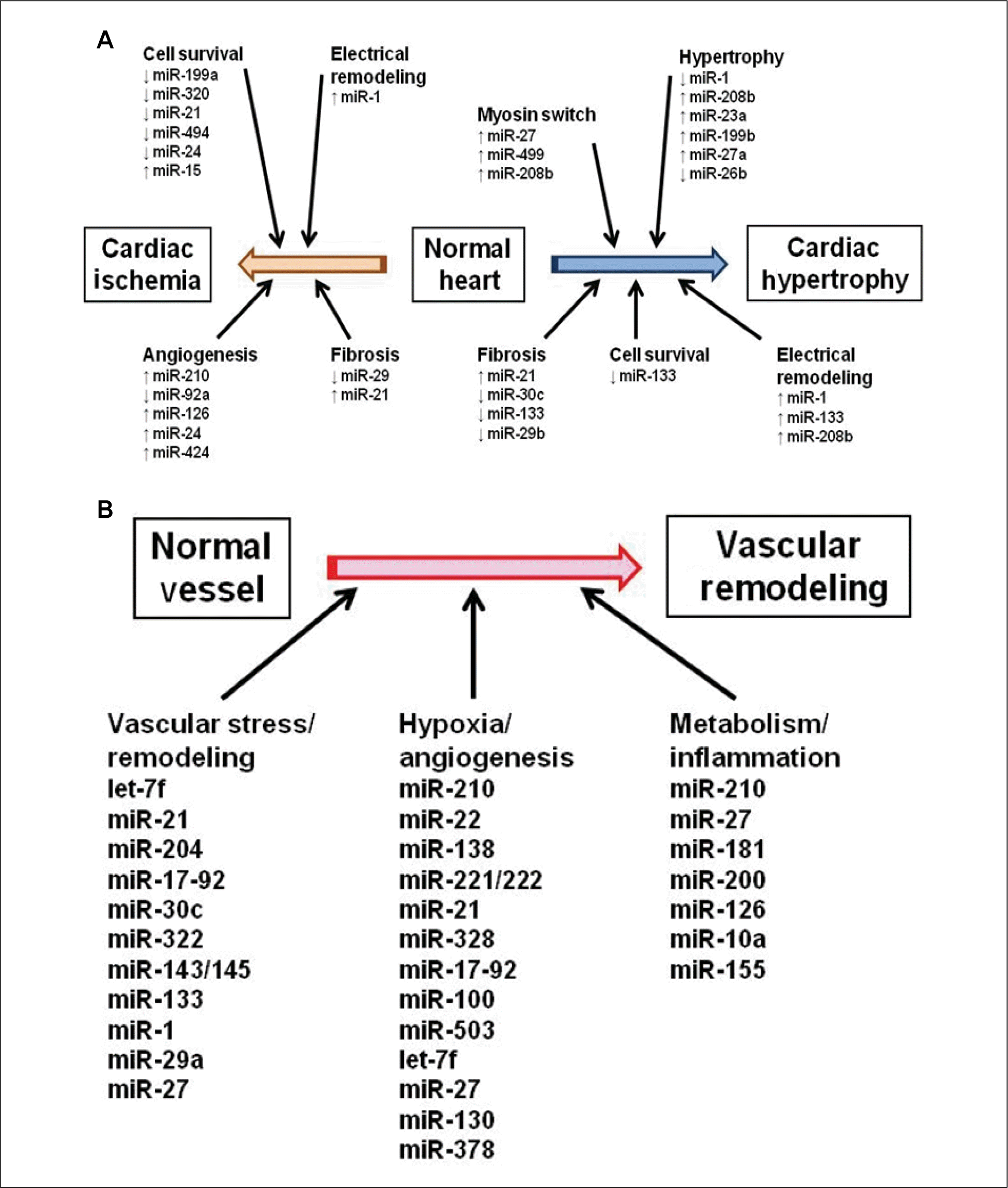
Fig. 3.
Pathobiology of pulmonary hypertension. Multiple vascular cell types in the pulmonary arterial wall and pulmonary arterial circulation are involved in the pathobiology of pulmonary arterial hypertension (PAH). Vascular pathology in PAH is triggered by various genetic and environmental stimuli, and initial injury to the endothelium and/or adventitial fibroblasts may activate pathogenic signaling pathways. These result in an imbalance of secreted vascular effectors that cause excessive pulmonary vasoconstriction and abnormal vascular remodeling processes. Common histological features in PAH include intimal hyperplasia, medial hypertrophy, adventitial proliferation/fibrosis, occlusion of small arteries, thrombosis in situ, and infiltration of inflammatory cells (green arrow) or progenitor cells (purple arrow). Transdifferentiation of endothelial cells or fibroblasts to vascular smooth muscle cells may contribute as well (blue arrow). These vascular changes lead to the formation of a layer of“neointima”(red arrow) and, in some cases, plexiform lesions (From Chan SY, et al. J Mol Cell Cardiol. 2008;44:14-30, with permission of PubMed Central).34)
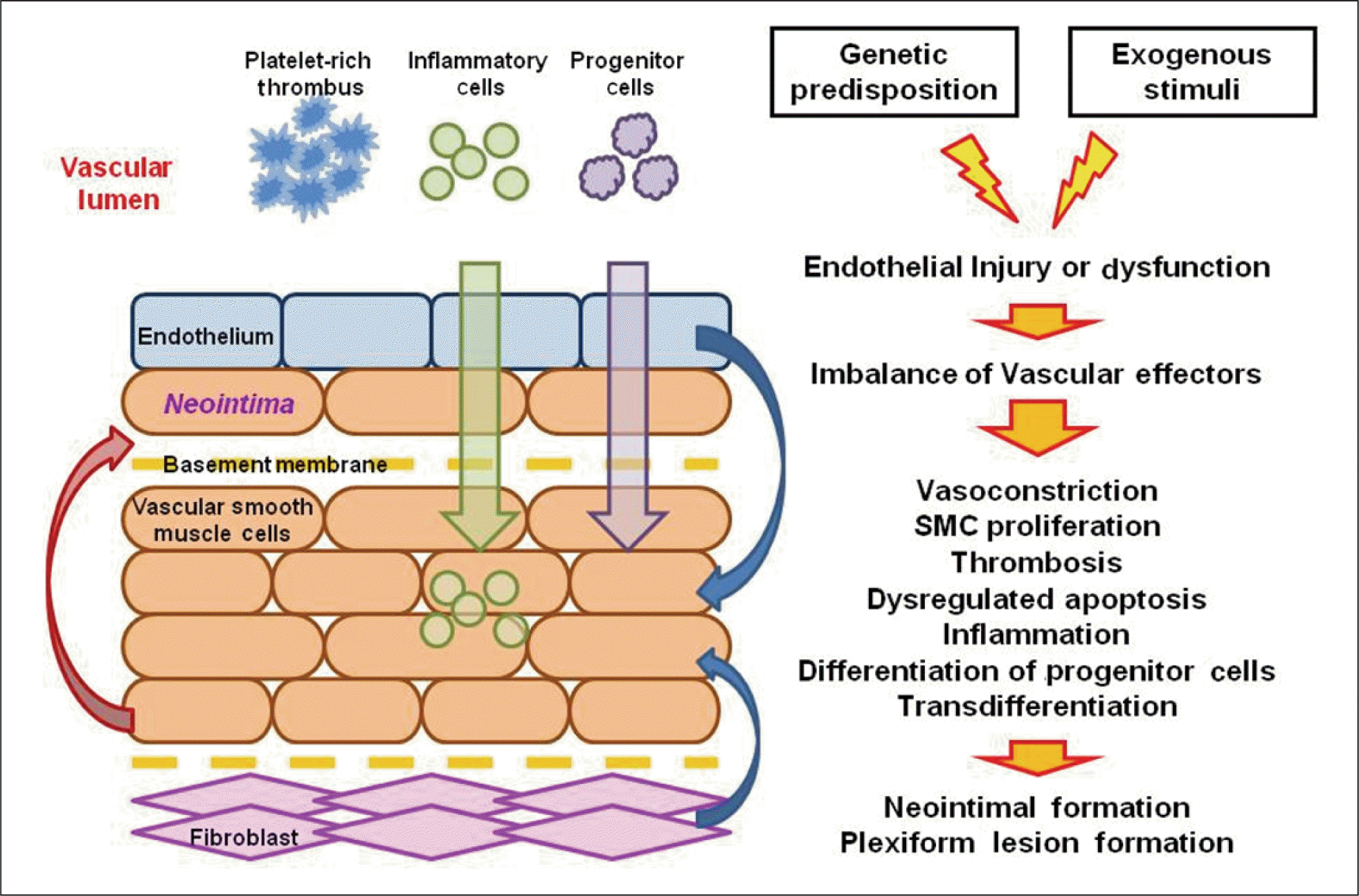
Fig. 4.
Pathogenic mechanisms of pulmonary hypertension (PH). Complex, pathogenic mechanisms that connect genetic predisposition, acquired, and exogenous factors play a role in the downstream dysfunction/dysregulation metabolism and signaling pathways resulting in PH.
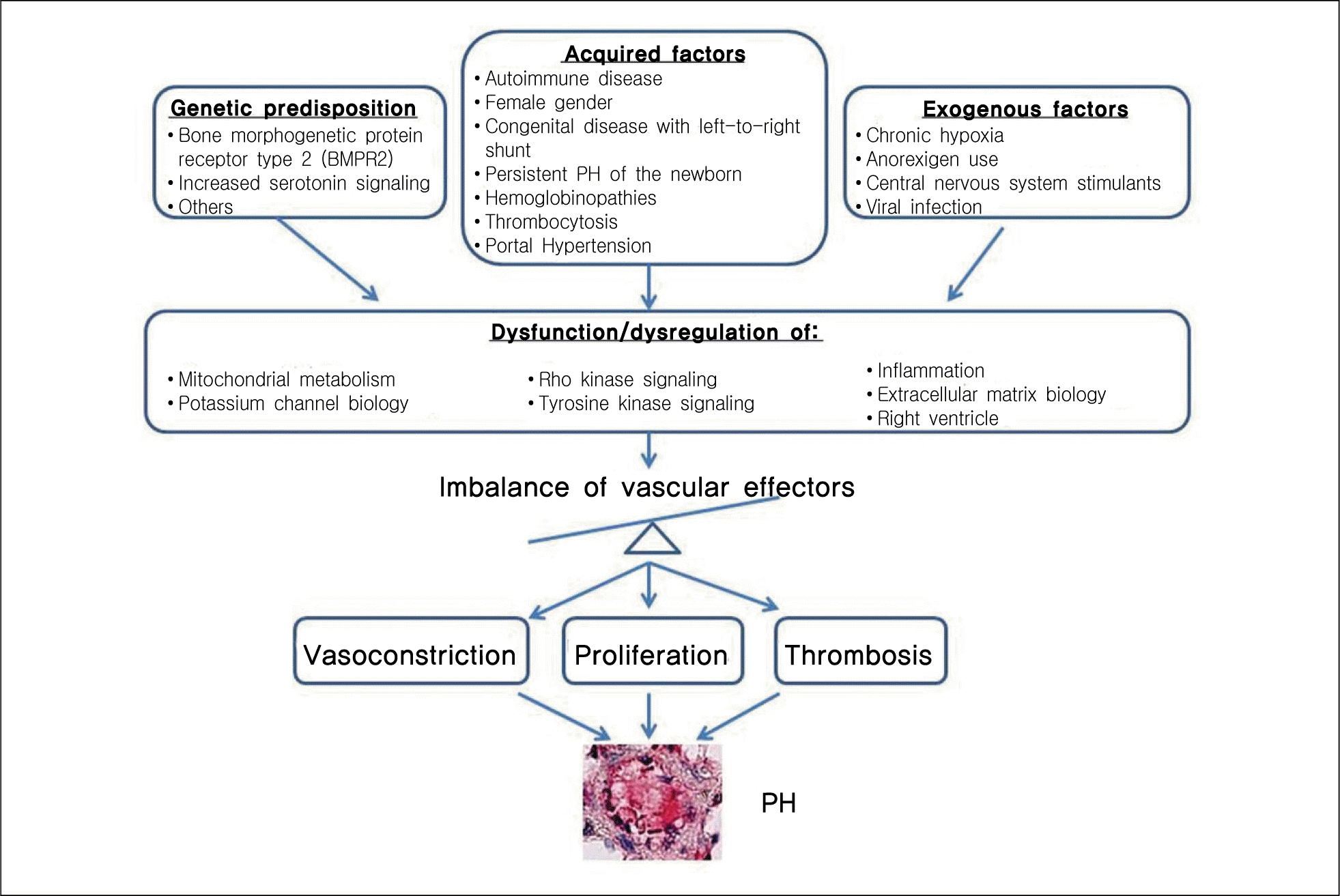
Fig. 5.
Roles of vascular effectors in the pathogenesis of pulmonary arterial hypertension (PAH). Vascular effectors have been shown to have different roles in the pathogenesis of pulmonary hypertension (PH) (From Chan SY, et al. J Mol Cell Cardiol. 2008;44:14-30, with permission of PubMed Central).34)
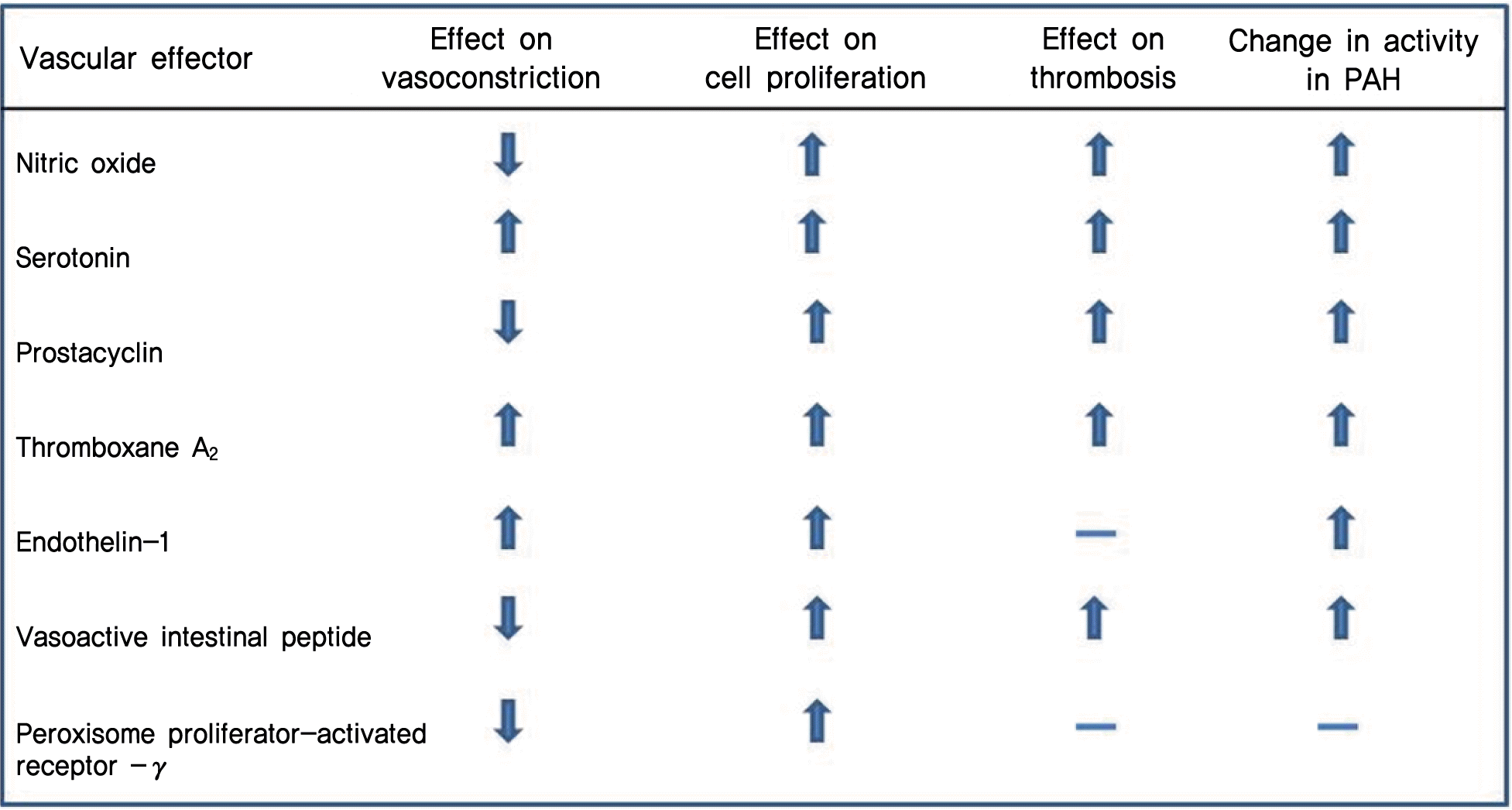
Fig. 6.
Validated actions of microRNA (miRNA) in pulmonary hypertension (PH). MiRNA are listed that carry confirmed functions in controlling PH in rodents and humans, as categorized by protective or pathogenic roles. Down-regulation of miR-145 protects against the development of pulmonary arterial hypertension (PAH), possibly through attenuation of myocardin expression.49) MiR-328 directly represses L-type calcium channel-α1C expression, resulting in reduction of pulmonary vasoconstrictive properties. Down-regulation of insulin growth factor 1 receptor may also contribute to pulmonary artery smooth muscle cell apoptosis.50) MiR-21 down-regulates RhoB and Rho-kinase activity to protect against mechanisms driving PAH.41) An antagomir directed against miR-20a restored functional bone morphogenetic protein receptor type 2 (BMPR2) signaling preventing PAH.48) For miR-17, the BMPR2 transcript is a predicted and validated target in cultured vascular cells.47) MiR-204 directly targets Src homology 2 domain-containing tyrosine phosphatase (SHP2) expression that inhibits nuclear factor of activated T cells (NFAT) expression playing a protective role against PAH.44) Down-regulation of miR-424 and miR-503 in PAH is associated with increased fibroblast growth factor 2 (FGF2) and fibroblast growth factor receptor 1 (FGFR1) expression.51) KLF4, Kruppel-like factor-4; IGF-1, insulin-like growth factor.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download