ABSTRACT
Background:
Hypertensive myocardial fibrosis promotes abnormalities of cardiac function that may adversely affect the clinical outcome of hypertensive patients. Imatinib mesylate blocks receptor tyrosine kinase and is clinically used to treat leukemia. Platelet-derived growth factor (PDGF) is a downstream target of receptor tyrosine kinases. Cardiac fibroblasts can be activated by PDGF. Thus we evaluated whether imatinib attenuate myocardial fibrosis and prevents diastolic dysfunction in spontaneously hypertensive rats (SHR).
Methods:
8 weeks old male SHRs were subjected to treatment with 8 weeks of low dose imatinib (SHR-10; 10 mg/kg), high dose imatinib (SHR-30; 30 mg/kg) or saline (SHR-C; n = 6 in each group). At the age of 16 weeks, all rats underwent hemodynamic studies and Doppler echocardiography, and were sacrificed. Their hearts were extracted for histopathological, immunoblotting and quantitative reverse transcriptase-polymerase chain reaction analyses.
Results:
While imatinib did not affect blood pressure (BP), it markedly reduced perivascular and interstitial fibrosis in the hearts of SHR. Echocardigram showed that high-dose imatinib significantly reduced left ventricular (LV) wall thickness (septal/posterior wall; SHR-C vs. SHR-30: 18 ± 2/19 ± 2 mm vs. 15 ± 1/14 ± 1 mm; p < 0.05) and improved the parameters of LV diastolic function such as E/A ratio (SHR-C vs. SHR-30: 1.60 ± 0.10 vs. 1.86 ± 0.20; p < 0.05). Imatinib also significantly reduced mRNA expression of collagen III and PDGF β-receptor tyrosine phosphorylation in the hearts of SHR.
References
1. Ruy KH, Han SW. Clinical characterisrics and prognositic factors of patients with congestive heart failure in Korea (epidemiology of congestive heart failure in Korea). J Korean Soc Hypertens. 2002; 8:38–47.
2. Kim TS, Youn HJ. From hypertension to heart failure. J Korean Soc Hypertens. 2009; 15:1–11.
3. Conrad CH, Brooks WW, Hayes JA, Sen S, Robinson KG, Bing OH. Myocardial fibrosis and stiffness with hypertrophy and heart failure in the spontaneously hypertensive rat. Circulation. 1995; 91:161–70.

4. Creemers EE, Pinto YM. Molecular mechanisms that control interstitial fibrosis in the pressure-overloaded heart. Cardiovasc Res. 2011; 89:265–72.

5. Rosenkranz S, Flesch M, Amann K, Haeuseler C, Kilter H, Seeland U, et al. Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta(1). Am J Physiol Heart Circ Physiol. 2002; 283:H1253–62.
6. Isoda K, Kamezawa Y, Tada N, Sato M, Ohsuzu F. Myocardial hypertrophy in transgenic mice overexpressing human interleukin 1alpha. J Card Fail. 2001; 7:355–64.
7. Bryant D, Becker L, Richardson J, Shelton J, Franco F, Peshock R, et al. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-alpha. Circulation. 1998; 97:1375–81.
8. Ponten A, Li X, Thoren P, Aase K, Sjoblom T, Ostman A, et al. Transgenic overexpression of platelet-derived growth factor-C in the mouse heart induces cardiac fibrosis, hypertrophy, and dilated cardiomyopathy. Am J Pathol. 2003; 163:673–82.
9. Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999; 79:1283–316.

10. Uutela M, Wirzenius M, Paavonen K, Rajantie I, He Y, Karpanen T, et al. PDGF-D induces macrophage recruitment, increased interstitial pressure, and blood vessel maturation during angiogenesis. Blood. 2004; 104:3198–204.

11. Fredriksson L, Li H, Eriksson U. The PDGF family: four gene products form five dimeric isoforms. Cytokine Growth Factor Rev. 2004; 15:197–204.

12. Savage DG, Antman KH. Imatinib mesylate–a new oral targeted therapy. N Engl J Med. 2002; 346:683–93.
13. Bing OH, Brooks WW, Robinson KG, Slawsky MT, Hayes JA, Litwin SE, et al. The spontaneously hypertensive rat as a model of the transition from compensated left ventricular hypertrophy to failure. J Mol Cell Cardiol. 1995; 27:383–96.

14. Cingolani OH, Yang XP, Liu YH, Villanueva M, Rhaleb NE, Carretero OA. Reduction of cardiac fibrosis decreases systolic performance without affecting diastolic function in hypertensive rats. Hypertension. 2004; 43:1067–73.

15. Daniels CE, Wilkes MC, Edens M, Kottom TJ, Murphy SJ, Limper AH, et al. Imatinib mesylate inhibits the profibro-genic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004; 114:1308–16.
16. Aono Y, Nishioka Y, Inayama M, Ugai M, Kishi J, Uehara H, et al. Imatinib as a novel antifibrotic agent in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med. 2005; 171:1279–85.

17. Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005; 201:925–35.

18. Distler JH, Jungel A, Huber LC, Schulze-Horsel U, Zwerina J, Gay RE, et al. Imatinib mesylate reduces production of extracellular matrix and prevents development of experimental dermal fibrosis. Arthritis Rheum. 2007; 56:311–22.

19. Soria A, Cario-Andre M, Lepreux S, Rezvani HR, Pasquet JM, Pain C, et al. The effect of imatinib (Glivec) on scleroderma and normal dermal fibroblasts: a preclinical study. Dermatology. 2008; 216:109–17.

20. Wang S, Wilkes MC, Leof EB, Hirschberg R. Imatinib mesylate blocks a non-Smad TGF-beta pathway and reduces renal fibrogenesis in vivo. FASEB J. 2005; 19:1–11.
21. Leipner C, Grun K, Muller A, Buchdunger E, Borsi L, Kosmehl H, et al. Imatinib mesylate attenuates fibrosis in coxsackievirus b3-induced chronic myocarditis. Cardiovasc Res. 2008; 79:118–26.

22. Schellings MW, Baumann M. van Leeuwen RE, Duisters RF, Janssen SH, Schroen B, et al. Imatinib attenuates end-organ damage in hypertensive homozygous TGR (mRen2)27 rats. Hypertension. 2006; 47:467–74.
23. Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007; 117:568–75.

24. Bhattacharyya S, Ishida W, Wu M, Wilkes M, Mori Y, Hinchcliff M, et al. A non-Smad mechanism of fibroblast activation by transforming growth factor-beta via c-Abl and Egr-1: selective modulation by imatinib mesylate. Oncogene. 2009; 28:1285–97.
25. Tuuminen R, Nykanen AI, Krebs R, Soronen J, Pajusola K, Keranen MA, et al. PDGF-A, -C, and -D but not PDGF-B increase TGF-beta1 and chronic rejection in rat cardiac allografts. Arterioscler Thromb Vasc Biol. 2009; 29:691–8.
26. Oudit GY, Penninger JM. Cardiac regulation by phosphoinositide 3-kinases and PTEN. Cardiovasc Res. 2009; 82:250–60.

27. Shimizu T, Kinugawa K, Yao A, Sugishita Y, Sugishita K, Harada K, et al. Platelet-derived growth factor induces cellular growth in cultured chick ventricular myocytes. Cardiovasc Res. 1999; 41:641–53.

28. Liu J, Wu LL, Li L, Zhang L, Song ZE. Growth-promoting effect of platelet-derived growth factor on rat cardiac myocytes. Regul Pept. 2005; 127:11–8.

29. Kagiyama S, Qian K, Kagiyama T, Phillips MI. Antisense to epidermal growth factor receptor prevents the development of left ventricular hypertrophy. Hypertension. 2003; 41:824–9.

30. Smith NJ, Chan HW, Osborne JE, Thomas WG, Hannan RD. Hijacking epidermal growth factor receptors by angiotensin II: new possibilities for understanding and treating cardiac hypertrophy. Cell Mol Life Sci. 2004; 61:2695–703.
31. Kerkela R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006; 12:908–16.

32. Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007; 7:332–44.

33. Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006; 355:2408–17.

34. Hochhaus A, Druker B, Sawyers C, Guilhot F, Schiffer CA, Cortes J, et al. Favorable long-term follow-up results over 6 years for response, survival, and safety with imatinib mesylate therapy in chronic-phase chronic myeloid leukemia after failure of interferon-alpha treatment. Blood. 2008; 111:1039–43.
35. Perik PJ, Rikhof B, de Jong FA, Verweij J, Gietema JA. van der Graaf WT. Results of plasma N-terminal pro B-type natriuretic peptide and cardiac troponin monitoring in GIST patients do not support the existence of imatinib-induced cardiotoxicity. Ann Oncol. 2008; 19:359–61.
36. Ribeiro AL, Marcolino MS, Bittencourt HN, Barbosa MM. Nunes Mdo C, Xavier VF, et al. An evaluation of the cardiotoxicity of imatinib mesylate. Leuk Res. 2008; 32:1809–14.
Fig. 1.
Light micrographs of the myocardium (×100, picrosirius red stain) reveal that the fibrosis of perivascular area and interstitial area is increased in SHR compared to WKY. The fibrosis of both areas was decreased after imatinib treatment in SHR (A, B). interstitial and perivascular area of WKY. (C, D). interstitial and perivascular area of SHR-control. (E, F). interstitial and perivascular area of SHR 10-mg. (G, H). interstitial and perivascular area of SHR 30-mg. WKY, Wistar Kyoto rats; SHR-control, spontaneously hypertensive rats treated with normal saline; SHR-10 mg, spontaneously hypertensive rats treated with 10 mg/kg of imatinib; SHR-30 mg, spontaneously hypertensive rats treated with 30 mg/kg of imatinib.

Fig. 2.
Collagen volume fraction of Left ventricle shows that the fibrosis of perivascular area and interstitial area is increased in SHR compared to WKY. Treatment with 30 mg/kg of imatinib in SHR significantly reduced the fibrosis of perivascular area and interstitial area. (A) Interstitial area. (B) Perivascular area. WKY, Wistar Kyoto rats; SHR-control, spontaneously hypertensive rats treated with normal saline; SHR-10 mg, spontaneously hypertensive rats treated with 10 mg/kg of imatinib; SHR-30 mg, spontaneously hypertensive rats treated with 30 mg/kg of imatinib. *p<0.05 vs. WKY. †p<0.05 vs. SHR.
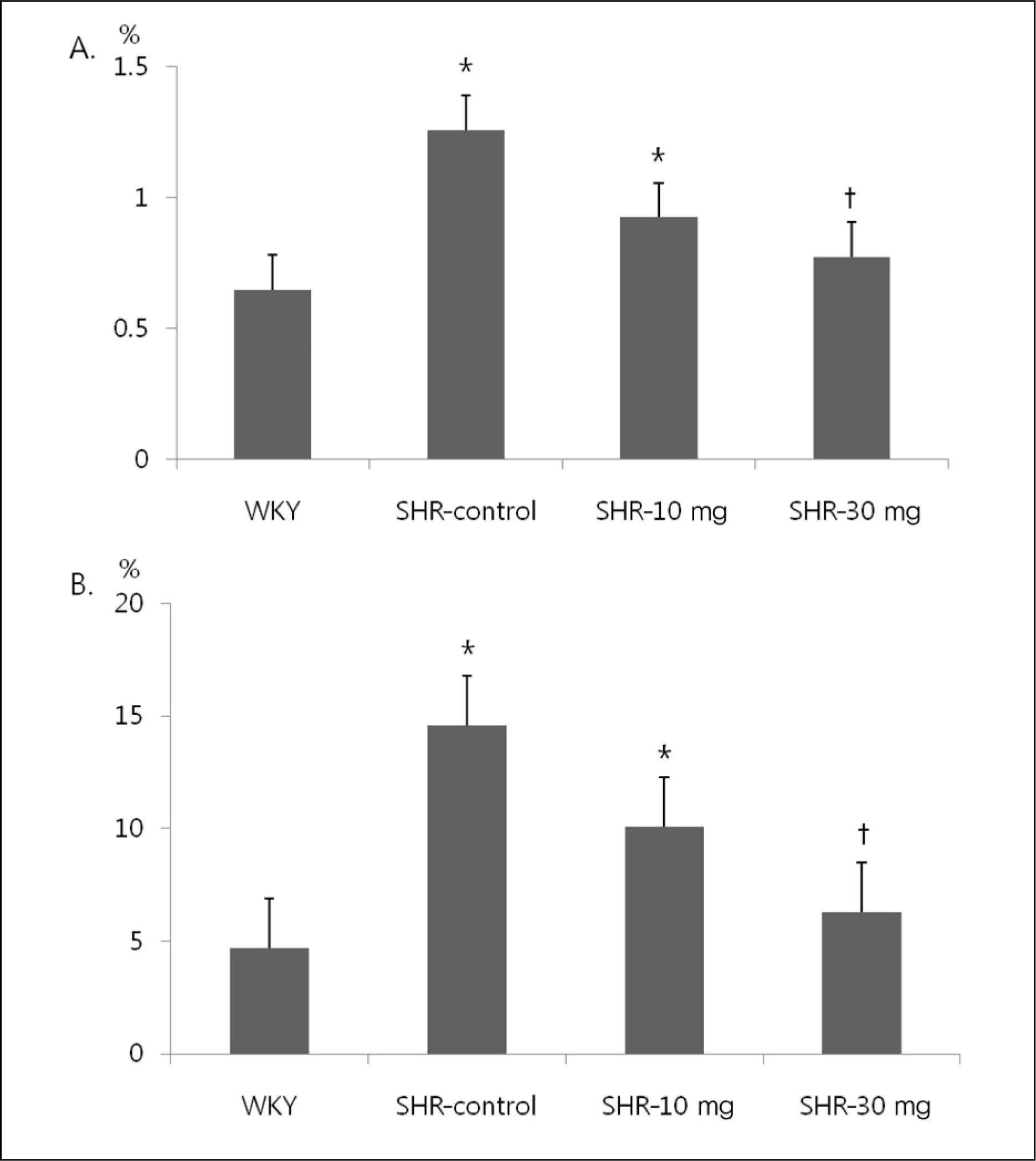
Fig. 3.
Myocardial expression of mRNA of collagen type III was increased in SHR compared to WKY. Expression of collagen type III was significantly decreased after treatment with 30 mg/kg of imatinib. Normalization relative GAPDH was performed. (A) Electrophoresis. (B) Expression of collagen type III. GAPDH, glyceride 3-phosphate dohydroqenase; WKY, Wistar Kyoto rats; SHR-control, spontaneously hypertensive rats treated with normal saline; SHR-10 mg, spontaneously hypertensive rats treated with 10 mg/kg of imatinib; SHR-30 mg, spontaneously hypertensive rats treated with 30 mg/kg of imatinib. *p<0.05 vs. WKY. †p<0.05 vs. SHR.
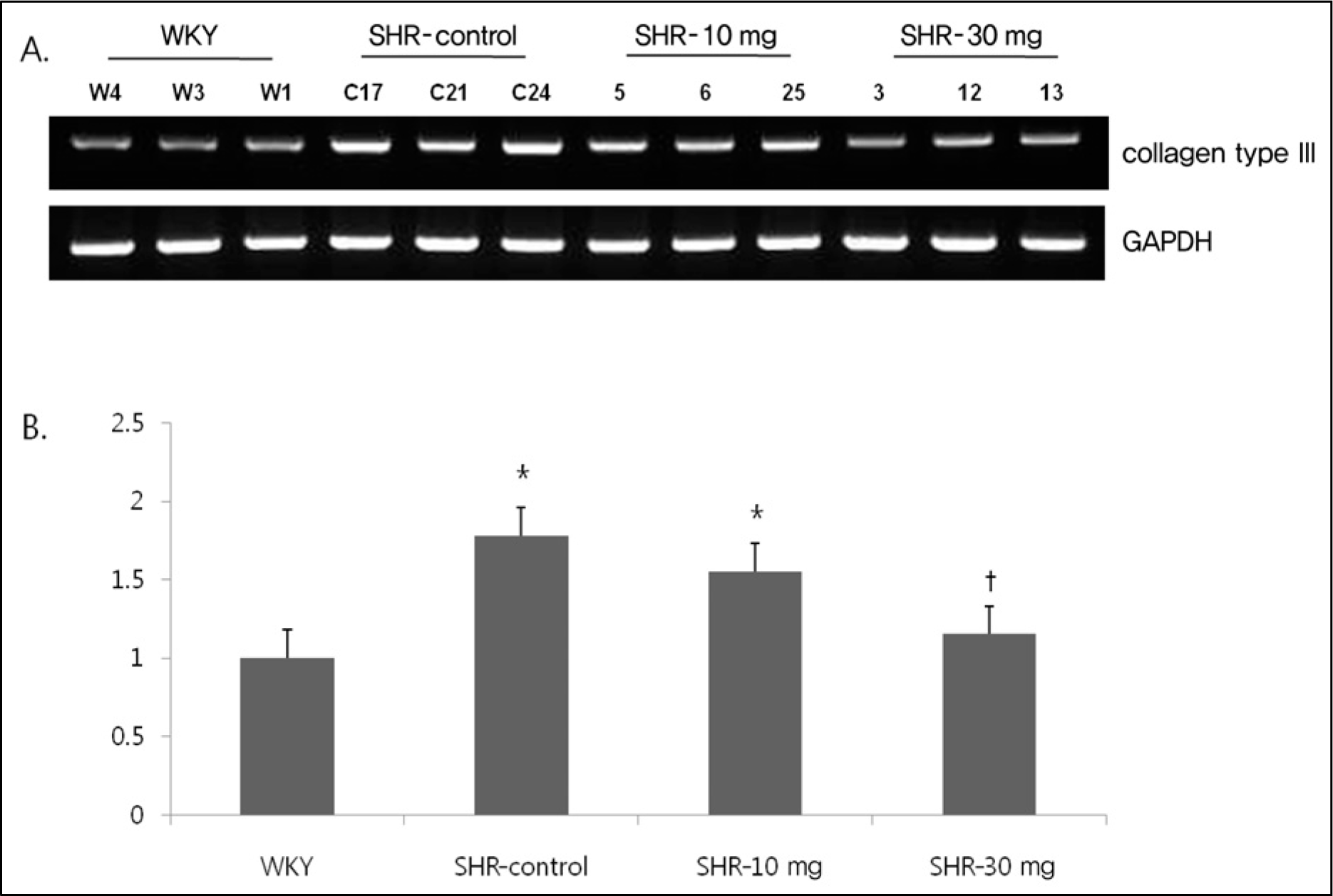
Fig. 4.
The effect of imatinib treatment on tyrosine phosphorylation level of platelet derived growth factor (PDGF)β . Tyrosine phosphorylation level of PDFGRβ was increased in SHR compared to WKY. Treatment with 30 mg/kg of imatinib decreased the tyrosine phosphorylation level of PDFGRβ . WKY, Wistar Kyoto rats; SHR-control, spontaneously hypertensive rats treated with normal saline; SHR-10 mg, spontaneously hypertensive rats treated with 10 mg/kg of imatinib; SHR-30 mg, spontaneously hypertensive rats treated with 30 mg/kg of imatinib.*p<0.05 vs. WKY. †p<0.05 vs. SHR.
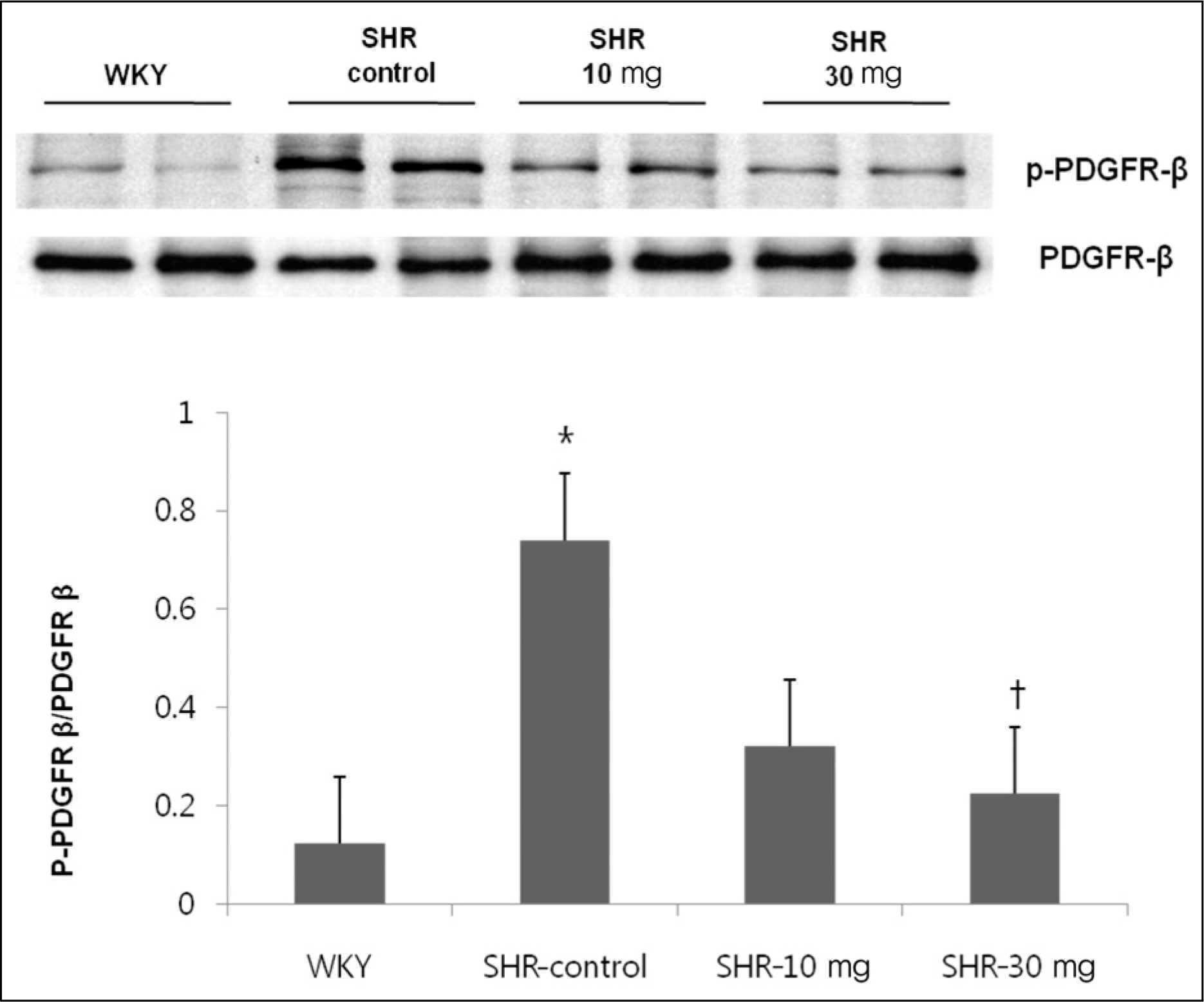
Table 1.
General characteristics
| WKY | SHR | SHR-10 mg | SHR-30 mg | |
|---|---|---|---|---|
| Pre-BWt (g) | 206 ±10 | 184 ±11* | 187 ±6* | 183 ±13* |
| Post-BWt (g) | 390 ±12 | 346 ±8* | 334 ±24* | 339 ±16* |
| Systolic BP (mm Hg) | 114 ±8 | 166 ±14* | 163 ±8* | 170 ±13* |
| Heart weight (g) | 1.2 ±0.1 | 1.5 ±0.1* | 1.3 ±0.1 | 1.4 ±0.1* |
| Tibia length (mm) | 5.2 ±0.4 | 4.8 ±0.2 | 4.8 ±0.3 | 4.8 ±0.2 |
| Heart/Tibia ratio (g/mm) | 0.23 ±0.01 | 0.31 ±0.03* | 0.28 ±0.02* | 0.30 ±0.02* |
Table 2.
Echocardiographic parameters
| WKY | SHR | SHR-10 mg | SHR-30 mg | |
|---|---|---|---|---|
| IVS (cm) | 0.12 ± 0.01 | 0.18 ± 0.02* | 0.17 ± 0.02* | 0.15 ± 0.01*†† |
| PW (cm) | 0.12 ± 0.01 | 0.19 ± 0.02* | 0.17 ± 0.02* | 0.14 ± 0.01†† |
| LVEDD (cm) | 0.64 ± 0.04 | 0.67 ± 0.04 | 0.66 ± 0.08 | 0.59 ± 0.10 |
| LVESD (cm) | 0.34 ± 0.02 | 0.40 ± 0.02* | 0.35 ± 0.06 | 0.33 ± 0.03 |
| FS (%) | 47 ± 2 | 44 ± 2 | 47 ± 3 | 47 ± 3 |
| E (m/sec) | 1.27 ± 0.08 | 1.08 ± 0.21 | 1.04 ± 0.23 | 1.09 ± 0.09 |
| A (m/sec) | 0.64 ± 0.06 | 0.68 ± 0.16 | 0.60 ± 0.16 | 0.59 ± 0.08 |
| HR (beat/min) | 313 ± 11 | 305 ± 12 | 307 ± 14 | 302 ± 8 |
| E/A | 1.97 ± 0.07 | 1.60 ± 0.10* | 1.78 ± 0.10 | 1.86 ± 0.20†† |
| DT (msec) | 29.7 ± 3.3 | 38.8 ± 2.2* | 35.8 ± 3.5* | 34 ± 3.7†† |
WKY, Wista Kyoto rat; SHR, spontaneous hypertensive rat; SHR-10 mg, SHR treated with 10 mg/kg of imatinib; SHR-30 mg, SHR treated with 30 mg/kg of imatinib; IVS, interventricular septal thickness; PW, posterior wall thickness; LVEDD, left ventricular end diastolic dimension; LVESD, left ventricular end systolic dimension; FS, fractional shortening of LV diameter; E, peak velocity of early transmitral inflow; A, peak velocity of late transmitral inflow; HR, heart rat; DT, deceleration time.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download