ABSTRACT
Transient receptor potential vanilloid type-1 channel (TRPV1) is a non-selective cation channel with a preference for calcium ions that is able to sense a vast range of endogenous physical and chemical stimuli and plays an important role in transducing the sensations of noxious heat and pain signaling. Recent studies showed that TRPV1 is widely expressed in different tissues and organs beyond the sensory nerves and has multiple biological effects that are involved in functional regulation in the pancreas, blood vessel, adipose tissue and liver. To further understand the link between TRPV1 and cardiometabolic diseases, we reviewed the role of TRPV1 in hypertension, diabetes, obesity, and dyslipidemia. This review provides new insights into the involvement of TRPV1 channels in the pathogenesis of cardiometabolic disorders and implicates this channel as a potential therapeutic target for the management of cardiometabolic diseases.
Go to : 
References
1. Wyne KL. Preventing cardiovascular disease and diabetes: a call to action from the ADA and AHA. J Cardiometab Syndr. 2006; 1:220–1.

2. Eckel RH, Kahn R, Robertson RM, Rizza RA. Preventing cardiovascular disease and diabetes: a call to action from the American Diabetes Association and the American Heart Association. Circulation. 2006; 113:2943–6.
3. Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, et al. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care. 2008; 31:811–22.
4. Plutzky J. Preventing type 2 diabetes and cardiovascular disease in metabolic syndrome: the role of PPARalpha. Diab Vasc Dis Res. 2007; 4(Suppl 3):S12–4.
5. American Heart Association Nutrition Committee , Lich-tenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006; 114:82–96.
6. Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010; 362:590–9.

7. Shin KO, Moritani T. Alterations of autonomic nervous activity and energy metabolism by capsaicin ingestion during aerobic exercise in healthy men. J Nutr Sci Vitaminol (Tokyo). 2007; 53:124–32.

8. Ahuja KD, Robertson IK, Geraghty DP, Ball MJ. Effects of chili consumption on postprandial glucose, insulin, and energy metabolism. Am J Clin Nutr. 2006; 84:63–9.

9. Wahlqvist ML, Wattanapenpaiboon N. Hot foods-unexpected help with energy balance? Lancet. 2001; 358:348–9.
10. Yang D, Luo Z, Ma S, Wong WT, Ma L, Zhong J, et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab. 2010; 12:130–41.

11. Zhang LL. Yan Liu D, Ma LQ, Luo ZD, Cao TB, Zhong J, et al. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res. 2007; 100:1063–70.
12. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997; 389:816–24.

13. Nagy I, Santha P, Jancso G, Urban L. The role of the va-nilloid (capsaicin) receptor (TRPV1) in physiology and pathology. Eur J Pharmacol. 2004; 500:351–69.

14. Sessa WC. A new way to lower blood pressure: pass the chili peppers please! Cell Metab. 2010; 12:109–10.

15. Gunthorpe MJ, Szallasi A. Peripheral TRPV1 receptors as targets for drug development: new molecules and mechanisms. Curr Pharm Des. 2008; 14:32–41.
16. Luo D, Zhang YW, Peng WJ, Peng J, Chen QQ, Li D, et al. Transient receptor potential vanilloid 1-mediated expression and secretion of endothelial cell-derived calcitonin gene-related peptide. Regul Pept. 2008; 150:66–72.

17. Sudhahar V, Shaw S, Imig JD. Mechanisms involved in oleamide-induced vasorelaxation in rat mesenteric resistance arteries. Eur J Pharmacol. 2009; 607:143–50.

18. Franco-Cereceda A. Resiniferatoxin-, capsaicin- and CGRP-evoked porcine coronary vasodilatation is independent of EDRF mechanisms but antagonized by CGRP(8-37). Acta Physiol Scand. 1991; 143:331–7.

20. Deng PY, Ye F, Cai WJ, Deng HW, Li YJ. Role of calcitonin gene-related peptide in the phenol-induced neurogenic hypertension in rats. Regul Pept. 2004; 119:155–61.

21. White CB, Roberts AM, Joshua IG. Arteriolar dilation mediated by capsaicin and calcitonin gene-related peptide in rats. Am J Physiol. 1993; 265:H1411–5.

22. Li J, Wang DH. High-salt-induced increase in blood pressure: role of capsaicin-sensitive sensory nerves. J Hyper-tens. 2003; 21:577–82.
23. Poblete IM, Orliac ML, Briones R, Adler-Graschinsky E, Huidobro-Toro JP. Anandamide elicits an acute release of nitric oxide through endothelial TRPV1 receptor activation in the rat arterial mesenteric bed. J Physiol. 2005; 568:539–51.

24. Bratz IN, Dick GM, Tune JD, Edwards JM, Neeb ZP, Dincer UD, et al. Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2008; 294:H2489–96.

25. Hao X, Chen J, Luo Z, He H, Yu H, Ma L, et al. TRPV1 activation prevents high-salt diet-induced nocturnal hypertension in mice. Pflugers Arch. 2011; 461:345–53.

26. Wang DH. The vanilloid receptor and hypertension. Acta Pharmacol Sin. 2005; 26:286–94.
27. Wang Y, Kaminski NE, Wang DH. Endocannabinoid regulates blood pressure via activation of the transient receptor potential vanilloid type 1 in Wistar rats fed a high-salt diet. J Pharmacol Exp Ther. 2007; 321:763–9.

28. Qin XP, Zeng SY, Li D, Chen QQ, Luo D, Zhang Z, et al. Calcitonin gene-related Peptide-mediated depressor effect and inhibiting vascular hypertrophy of rutaecarpine in renovascular hypertensive rats. J Cardiovasc Pharmacol. 2007; 50:654–9.

29. Wang Y, Babankova D, Huang J, Swain GM, Wang DH. Deletion of transient receptor potential vanilloid type 1 receptors exaggerates renal damage in deoxycorticosterone acetate-salt hypertension. Hypertension. 2008; 52:264–70.

30. Sexton A, McDonald M, Cayla C, Thiemermann C, Ahluwalia A. 12-Lipoxygenase-derived eicosanoids protect against myocardial ischemia/reperfusion injury via activation of neuronal TRPV1. FASEB J. 2007; 21:2695–703.
31. Wang L, Wang DH. TRPV1 gene knockout impairs postischemic recovery in isolated perfused heart in mice. Circulation. 2005; 112:3617–23.

32. Li YJ, Xiao ZS, Peng CF, Deng HW. Calcitonin gene-related peptide-induced preconditioning protects against ischemia-reperfusion injury in isolated rat hearts. Eur J Pharmacol. 1996; 311:163–7.

33. Huang W, Rubinstein J, Prieto AR, Thang LV, Wang DH. Transient receptor potential vanilloid gene deletion exacerbates inflammation and atypical cardiac remodeling after myocardial infarction. Hypertension. 2009; 53:243–50.

34. Zhong B, Wang DH. TRPV1 gene knockout impairs preconditioning protection against myocardial injury in isolated perfused hearts in mice. Am J Physiol Heart Circ Physiol. 2007; 293:H1791–8.

35. Akiba Y, Kato S, Katsube K, Nakamura M, Takeuchi K, Ishii H, et al. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet beta cells modulates insulin secretion in rats. Biochem Biophys Res Commun. 2004; 321:219–25.
36. Tolan I, Ragoobirsingh D, Morrison EY. The effect of cap-saicin on blood glucose, plasma insulin levels and insulin binding in dog models. Phytother Res. 2001; 15:391–4.

37. Kang JH, Kim CS, Han IS, Kawada T, Yu R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett. 2007; 581:4389–96.

38. Domotor A, Szolcsanyi J, Mozsik G. Capsaicin and glucose absorption and utilization in healthy human subjects. Eur J Pharmacol. 2006; 534:280–3.
39. van de Wall EH, Gram DX, Strubbe JH, Scheurink AJ, Koolhaas JM. Ablation of capsaicin-sensitive afferent nerves affects insulin response during an intravenous glucose tolerance test. Life Sci. 2005; 77:1283–92.

40. Karlsson S, Ahren B. Capsaicin-induced sensory denervation increases glucose elimination in rodents. Diabetologia. 1999; 42:260–1.

41. Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, et al. TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell. 2006; 127:1123–35.
42. Gram DX, Ahren B, Nagy I, Olsen UB, Brand CL, Sundler F, et al. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur J Neurosci. 2007; 25:213–23.
43. Gram DX, Hansen AJ, Deacon CF, Brand CL, Ribel U, Wilken M, et al. Sensory nerve desensitization by resiniferatoxin improves glucose tolerance and increases insulin secretion in Zucker Diabetic Fatty rats and is associated with reduced plasma activity of dipeptidyl peptidase IV. Eur J Pharmacol. 2005; 509:211–7.

44. Suri A, Szallasi A. The emerging role of TRPV1 in diabetes and obesity. Trends Pharmacol Sci. 2008; 29:29–36.

45. Guillot E, Coste A, Angel I. Involvement of capsaicin-sensitive nerves in the regulation of glucose tolerance in diabetic rats. Life Sci. 1996; 59:969–77.

46. Xin H, Tanaka H, Yamaguchi M, Takemori S, Nakamura A, Kohama K. Vanilloid receptor expressed in the sarcoplasmic reticulum of rat skeletal muscle. Biochem Biophys Res Commun. 2005; 332:756–62.

47. Castro J, Aromataris EC, Rychkov GY, Barritt GJ. A small component of the endoplasmic reticulum is required for store-operated Ca2+ channel activation in liver cells: evidence from studies using TRPV1 and taurodeoxycholic acid. Biochem J. 2009; 418:553–66.

48. Manjunatha H, Srinivasan K. Hypolipidemic and antioxidant effects of curcumin and capsaicin in high-fat-fed rats. Can J Physiol Pharmacol. 2007; 85:588–96.

49. Tani Y, Fujioka T, Sumioka M, Furuichi Y, Hamada H, Watanabe T. Effects of capsinoid on serum and liver lipids in hyperlipidemic rats. J Nutr Sci Vitaminol (Tokyo). 2004; 50:351–5.

50. Lee CY, Kim M, Yoon SW, Lee CH. Short-term control of capsaicin on blood and oxidative stress of rats in vivo. Phytother Res. 2003; 17:454–8.
51. Kawada T, Hagihara K, Iwai K. Effects of capsaicin on lipid metabolism in rats fed a high fat diet. J Nutr. 1986; 116:1272–8.

52. Kahn-Kirby AH, Dantzker JL, Apicella AJ, Schafer WR, Browse J, Bargmann CI, et al. Specific polyunsaturated fatty acids drive TRPV-dependent sensory signaling in vivo. Cell. 2004; 119:889–900.

53. Matta JA, Miyares RL, Ahern GP. TRPV1 is a novel target for omega-3 polyunsaturated fatty acids. J Physiol. 2007; 578:397–411.

54. Liu M, Huang W, Wu D, Priestley JV. TRPV1, but not P2X, requires cholesterol for its function and membrane expression in rat nociceptors. Eur J Neurosci. 2006; 24:1–6.
55. Melnyk A, Himms-Hagen J. Resistance to aging-associated obesity in capsaicin-desensitized rats one year after treatment. Obes Res. 1995; 3:337–44.

56. Herman RM, Brower JB, Stoddard DG, Casano AR, Targovnik JH, Herman JH, et al. Prevalence of somatic small fiber neuropathy in obesity. Int J Obes (Lond). 2007; 31:226–35.

57. Motter AL, Ahern GP. TRPV1-null mice are protected from diet-induced obesity. FEBS Lett. 2008; 582:2257–62.

58. Pare M, Albrecht PJ, Noto CJ, Bodkin NL, Pittenger GL, Schreyer DJ, et al. Differential hypertrophy and atrophy among all types of cutaneous innervation in the glabrous skin of the monkey hand during aging and naturally occurring type 2 diabetes. J Comp Neurol. 2007; 501:543–67.
59. Kawada T, Watanabe T, Takaishi T, Tanaka T, Iwai K. Capsaicin-induced beta-adrenergic action on energy metabolism in rats: influence of capsaicin on oxygen consumption, the respiratory quotient, and substrate utilization. Proc Soc Exp Biol Med. 1986; 183:250–6.
60. Kim KM, Kawada T, Ishihara K, Inoue K, Fushiki T. Increase in swimming endurance capacity of mice by capsaicin-induced adrenal catecholamine secretion. Biosci Biotechnol Biochem. 1997; 61:1718–23.

61. Ohnuki K, Haramizu S, Oki K, Watanabe T, Yazawa S, Fushiki T. Administration of capsiate, a non-pungent capsaicin analog, promotes energy metabolism and suppresses body fat accumulation in mice. Biosci Biotechnol Bio-chem. 2001; 65:2735–40.

62. Leung FW. Capsaicin-sensitive intestinal mucosal afferent mechanism and body fat distribution. Life Sci. 2008; 83:1–5.

63. Inoue N, Matsunaga Y, Satoh H, Takahashi M. Enhanced energy expenditure and fat oxidation in humans with high BMI scores by the ingestion of novel and non-pungent capsaicin analogues (capsinoids). Biosci Biotechnol Biochem. 2007; 71:380–9.

64. Wang X, Miyares RL, Ahern GP. Oleoylethanolamide excites vagal sensory neurones, induces visceral pain and reduces short-term food intake in mice via capsaicin receptor TRPV1. J Physiol. 2005; 564:541–7.

Go to : 
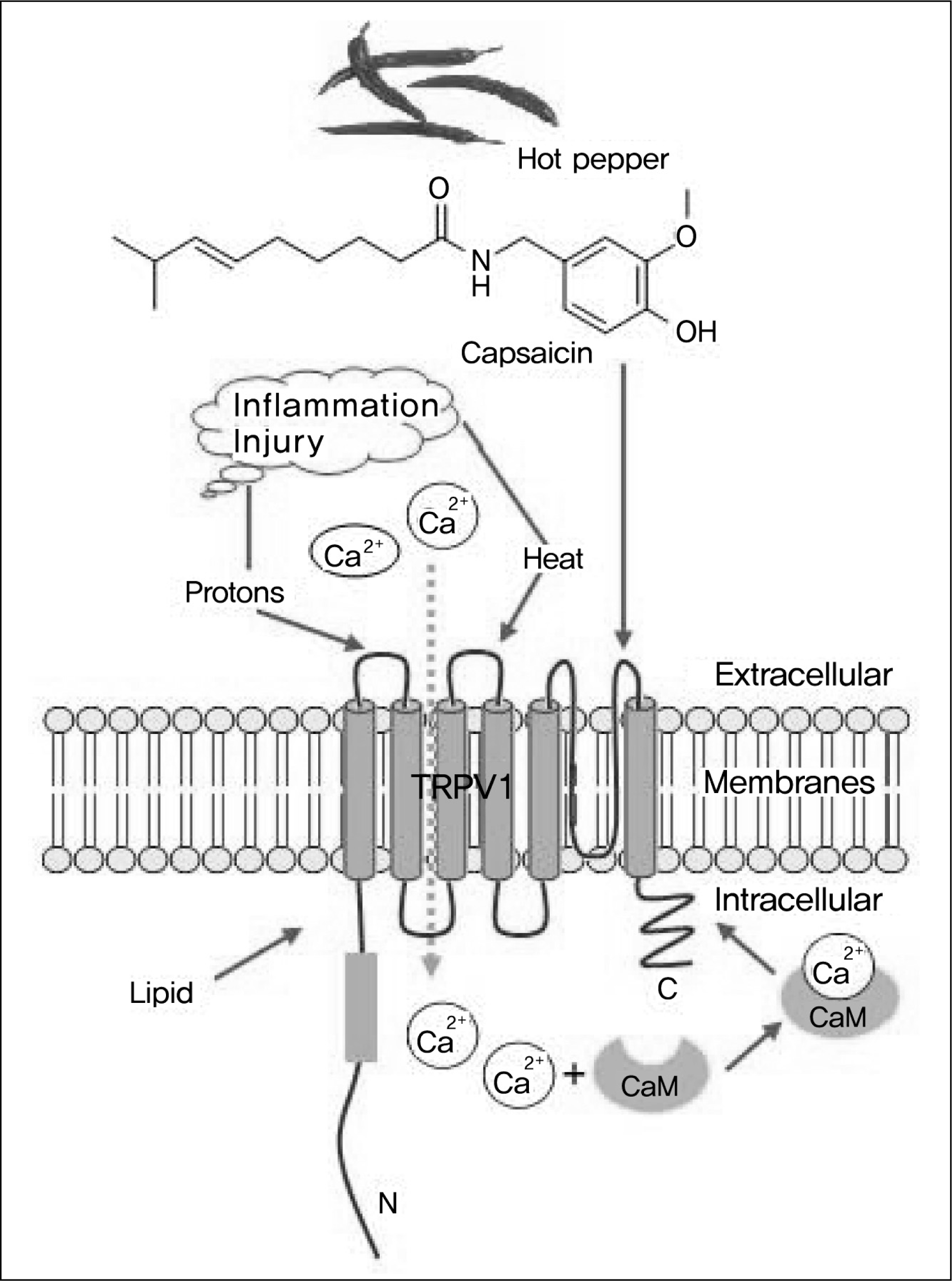 | Fig. 1.
Transient receptor potential vanilloid type-1 channel (TRPV1) has six transmembrane domains and a short, pore-forming hydrophobic stretch between the fifth and sixth transmembrane domains. Capsaicin binding to TRPV1 triggers an increase in intracellular calcium. When this occurs in sensory nerves, it promotes the sensation of pain, inflammation, and local heat. TRPV1 is also activated by noxious heat (>43°C) and acid (pH<5.9), voltage, and various lipids. |
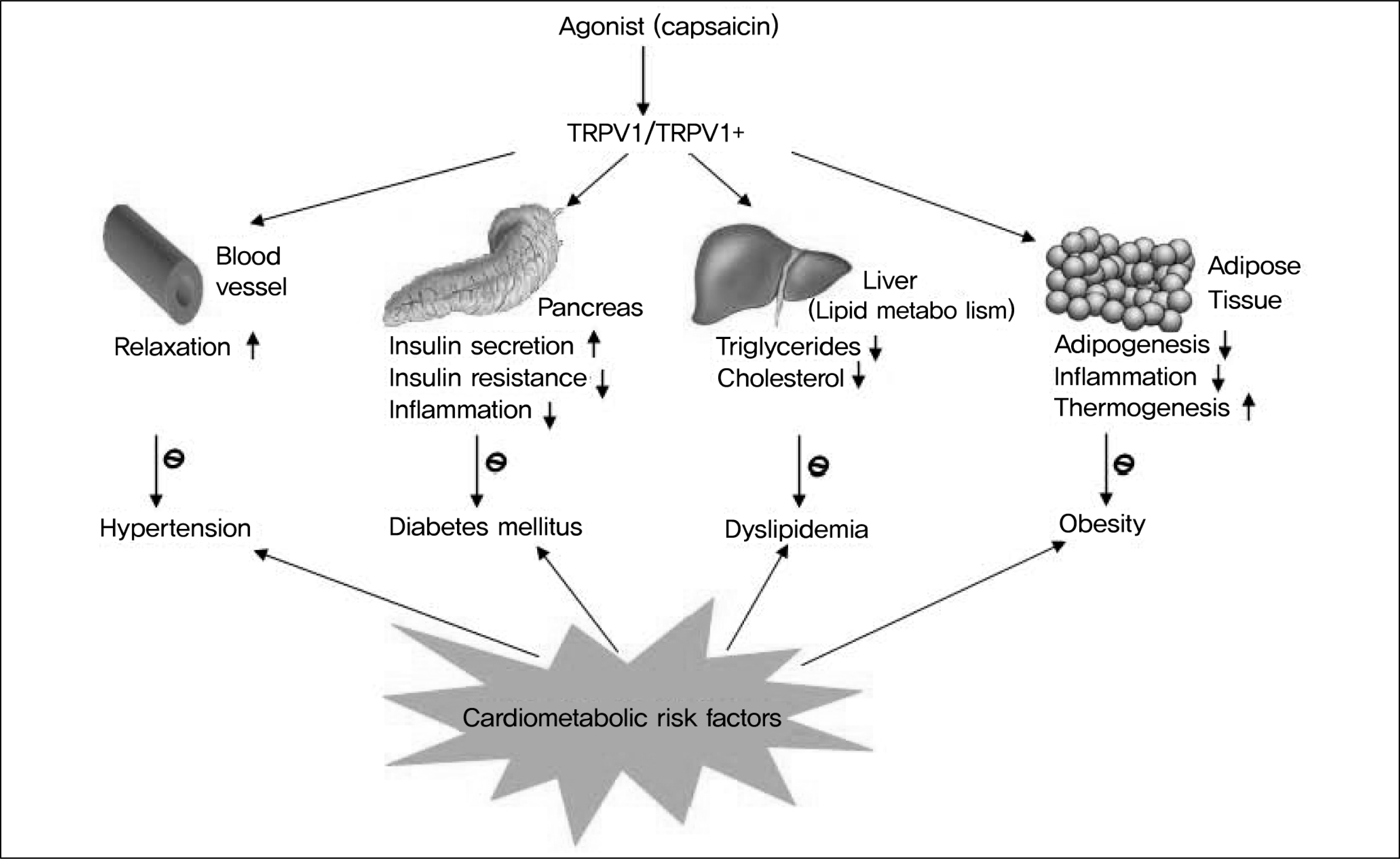 | Fig. 2.
Transient receptor potential vanilloid type-1 channel (TRPV1) is widely expressed in the car-diometabolic system such as in pancreas, blood vessels, adipose tissue and liver. Activation of TRPV1 causes the relaxation of arteries leading to a decrease in blood pressure; increases the secretion of insulin from islet β cells and lowers blood glucose; improves insulin resistance; reduces inflammation, triglycerides, cholesterol, and adipogenesis; increases thermogenesis; and prevents obesity, implying that activation of TRPV1 will decrease cardiometabolic risk. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download