ABSTRACT
Cyclophilin A (CyPA) is a 17 kDa, ubiquitously expressed multifunctional protein that possesses peptidylprolyl cis-trans isomerase activity and scaffold function. Its expression is increased in inflammatory conditions including rheumatoid arthritis, autoimmune disease and cancer. Intracellular CyPA regulates protein trafficking, signal transduction, transcription regulation and the activity of certain other proteins. Secreted CyPA activates cardiovascular cells resulting in a variety of cardiovascular diseases; including vascular remodeling, abdominal aortic aneurysms formation, atherosclerosis, cardiac hypertrophy and myocardial ischemic reperfusion injury.
References
1. Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003; 42:1075–81.
2. Griendling KK, Ushio-Fukai M. Redox control of vascular smooth muscle proliferation. J Lab Clin Med. 1998; 132:9–15.

3. Berk BC. Atheroprotective signaling mechanisms activated by steady laminar flow in endothelial cells. Circulation. 2008; 117:1082–9.

4. Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994; 74:1141–8.

5. Frey RS, Masuko U-F, Malik AB. Forum review NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal. 2009; 11:791–810.
6. Rathore R, Zheng YM, Niu CF, Liu QH, Korde A, Ho YS, et al. Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PK Cepsilon signaling axis in pulmonary artery smooth muscle cells. Free Radic Biol Med. 2008; 45:1223–31.
7. Birukov KG. Cyclic stretch, reactive oxygen species, and vascular remodeling. Antioxid Redox Signal. 2009; 11:1651–67.

9. Ryffel B, Woerly G, Greiner B, Haendler B, Mihatsch MJ, Foxwell BM. Distribution of the cyclosporine binding protein cyclophilin in human tissues. Immunology. 1991; 72:399–404.
10. Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984; 226:544–7.

11. Takahashi N, Hayano T, Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989; 337:473–5.

12. Schreiber SL. Chemistry and biology of the im-munophilins and their immunosuppressive ligands. Science. 1991; 251:283–7.

13. Zhu C, Wang X, Deinum J, Huang Z, Gao J, Modjtahedi N, et al. Cyclophilin A participates in the nuclear translocation of apoptosis-inducing factor in neurons after cerebral hypoxia-ischemia. J Exp Med. 2007; 204:1741–8.

14. Brazin KN, Mallis RJ, Fulton DB, Andreotti AH. Regulation of the tyrosine kinase Itk by the peptidyl-prolyl isomerase cyclophilin A. Proc Natl Acad Sci U S A. 2002; 99:1899–904.

15. Colgan J, Asmal M, Neagu M, Yu B, Schneidkraut J, Lee Y, et al. Cyclophilin A regulates TCR signal strength in CD4+ T cells via a proline-directed conformational switch in Itk. Immunity. 2004; 21:189–201.

16. Krummrei U, Bang R, Schmidtchen R, Brune K, Bang H. Cyclophilin-A is a zinc-dependent DNA binding protein in macrophages. FEBS Lett. 1995; 371:47–51.

17. Walter DH, Haendeler J, Galle J, Zeiher AM, Dimmeler S. Cyclosporin A inhibits apoptosis of human endothelial cells by preventing release of cytochrome C from mitochondria. Circulation. 1998; 98:1153–7.

18. Jonasson L, Holm J, Hansson GK. Cyclosporin A inhibits smooth muscle proliferation in the vascular response to injury. Proc Natl Acad Sci U S A. 1988; 85:2303–6.

19. Gregory CR, Huang X, Pratt RE, Dzau VJ, Shorthouse R, Billingham ME, et al. Treatment with rapamycin and mycophenolic acid reduces arterial intimal thickening produced by mechanical injury and allows endothelial replacement. Transplantation. 1995; 59:655–61.

20. Andersen HO, Hansen BF, Holm P, Stender S, Nordestgaard BG. Effect of cyclosporine on arterial balloon injury lesions in cholesterol-clamped rabbits: T lymphocyte-mediated immune responses not involved in balloon injury-induced neointimal proliferation. Arterioscler Thromb Vasc Biol. 1999; 19:1687–94.
21. Ferns G, Reidy M, Ross R. Vascular effects of cyclosporine A in vivo and in vitro. Am J Pathol. 1990; 137:403–13.
22. Jin ZG, Lungu AO, Xie L, Wang M, Wong C, Berk BC. Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler Thromb Vasc Biol. 2004; 24:1186–91.

23. Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, Suh YA, et al. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res. 2000; 87:789–96.

24. Satoh K, Matoba T, Suzuki J, O’Dell MR, Nigro P, Cui Z, et al. Cyclophilin A mediates vascular remodeling by promoting inflammation and vascular smooth muscle cell proliferation. Circulation. 2008; 117:3088–98.

25. Sun J, Hemler ME. Regulation of MMP-1 and MMP-2 production through CD147/extracellular matrix metal-loproteinase inducer interactions. Cancer Res. 2001; 61:2276–81.
26. Pushkarsky T, Zybarth G, Dubrovsky L, Yurchenko V, Tang H, Guo H, et al. CD147 facilitates HIV-1 infection by interacting with virus-associated cyclophilin A. Proc Natl Acad Sci U S A. 2001; 98:6360–5.

27. Damsker JM, Bukrinsky MI, Constant SL. Preferential chemotaxis of activated human CD4+ T cells by extracellular cyclophilin A. J Leukoc Biol. 2007; 82:613–8.
28. Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000; 6:435–42.

29. Pakula R, Melchior A, Denys A, Vanpouille C, Mazurier J, Allain F. Syndecan-1/CD147 association is essential for cyclophilin B-induced activation of p44/42 mitogen- activated protein kinases and promotion of cell adhesion and chemotaxis. Glycobiology. 2007; 17:492–503.
30. Hanoulle X, Melchior A, Sibille N, Parent B, Denys A, Wieruszeski JM, et al. Structural and functional characterization of the interaction between Cyclophilin B and a heparin-derived oligosaccharide. J Biol Chem. 2007; 282:34148–58.

31. Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000; 1:151–5.

32. Pushkarsky T, Yurchenko V, Laborico A, Bukrinsky M. CD147 stimulates HIV-1 infection in a signal-in-dependent fashion. Biochem Biophys Res Commun. 2007; 363:495–9.

33. Suzuki J, Jin ZG, Meoli DF, Matoba T, Berk BC. Cyclophilin A is secreted by a vesicular pathway in vascular smooth muscle cells. Circ Res. 2006; 98:811–7.

34. Colgan J, Asmal M, Yu B, Luban J. Cyclophilin A-deficient mice are resistant to immunosuppression by cyclosporine. J Immunol. 2005; 174:6030–8.

35. Miller AT, Wilcox HM, Lai Z, Berg LJ. Signaling through Itk promotes T helper 2 differentiation via negative regulation of T-bet. Immunity. 2004; 21:67–80.

36. Zhou X, Stemme S, Hansson GK. Evidence for a local immune response in atherosclerosis. CD4+ T cells infiltrate lesions of apolipoprotein-E-deficient mice. Am J Pathol. 1996; 149:359–66.
37. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005; 352:1685–95.

38. Xu Q. Role of heat shock proteins in atherosclerosis. Arterioscler Thromb Vasc Biol. 2002; 22:1547–59.

39. Al-Daraji WI, Grant KR, Ryan K, Saxton A, Reynolds NJ. Localization of calcineurin/NFAT in human skin and psoriasis and inhibition of calcineurin/NFAT activation in human keratinocytes by cyclosporin A. J Invest Dermatol. 2002; 118:779–88.

40. Arevalo-Rodriguez M, Heitman J. Cyclophilin A is localized to the nucleus and controls meiosis in Saccharomyces cerevisiae. Eukaryot Cell. 2005; 4:17–29.
41. Pan H, Luo C, Li R, Qiao A, Zhang L, Mines M, et al. Cyclophilin A is required for CXCR4-mediated nuclear export of heterogeneous nuclear ribonucleoprotein A2, activation and nuclear translocation of ERK1/2, and chemotactic cell migration. J Biol Chem. 2008; 283:623–37.

42. Sherry B, Yarlett N, Strupp A, Cerami A. Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc Natl Acad Sci U S A. 1992; 89:3511–5.

43. Rietschel ET, Schletter J, Weidemann B, El-Samalouti V, Mattern T, Zahringer U, et al. Lipopolysaccharide and peptidoglycan: CD14-dependent bacterial inducers of inflammation. Microb Drug Resist. 1998; 4:37–44.

44. Fujihara M, Muroi M, Tanamoto K, Suzuki T, Azuma H, Ikeda H. Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: roles of the receptor complex. Pharmacol Ther. 2003; 100:171–94.

45. Billich A, Winkler G, Aschauer H, Rot A, Peichl P. Presence of cyclophilin A in synovial fluids of patients with rheumatoid arthritis. J Exp Med. 1997; 185:975–80.

46. Tegeder I, Schumacher A, John S, Geiger H, Geisslinger G, Bang H, et al. Elevated serum cyclophilin levels in patients with severe sepsis. J Clin Immunol. 1997; 17:380–6.
47. Endrich MM, Gehring H. The V3 loop of human immunodeficiency virus type-1 envelope protein is a high-affinity ligand for immunophilins present in human blood. Eur J Biochem. 1998; 252:441–6.

48. Liao DF, Jin ZG, Baas AS, Daum G, Gygi SP, Aebersold R, et al. Purification and identification of secreted oxidative stress-induced factors from vascular smooth muscle cells. J Biol Chem. 2000; 275:189–96.

49. Baas AS, Berk BC. Differential activation of mitogen-activated protein kinases by H2O2 and O2- in vascular smooth muscle cells. Circ Res. 1995; 77:29–36.
50. Meloche S, Seuwen K, Pages G, Pouyssegur J. Biphasic and synergistic activation of p44mapk (ERK1) by growth factors: correlation between late phase activation and mitogenicity. Mol Endocrinol. 1992; 6:845–54.

51. York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, et al. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998; 392:622–6.

52. Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, et al. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998; 32:488–95.
53. Rajagopalan S, Kurz S, Munzel T, Tarpey M, Freeman BA, Griendling KK, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996; 97:1916–23.

54. Satoh K, Nigro P, Matoba T, O’Dell MR, Cui Z, Shi X, et al. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat Med. 2009; 15:649–56.

55. Takapoo M, Chamseddine AH, Bhalla RC, Miller FJ Jr. Glutathione peroxidase-deficient smooth muscle cells cause paracrine activation of normal smooth muscle cells via cyclophilin A. Vascul Pharmacol. 2011; 55:143–8.

56. Kim SH, Lessner SM, Sakurai Y, Galis ZS. Cyclophilin A as a novel biphasic mediator of endothelial activation and dysfunction. Am J Pathol. 2004; 164:1567–74.

57. Seko Y, Tobe K, Ueki K, Kadowaki T, Yazaki Y. Hypoxia and hypoxia/reoxygenation activate Raf-1, mitogen- activated protein kinase kinase, mitogen-activated protein kinases, and S6 kinase in cultured rat cardiac myocytes. Circ Res. 1996; 78:82–90.
58. Seko Y, Tobe K, Takahashi N, Kaburagi Y, Kadowaki T, Yazaki Y. Hypoxia and hypoxia/reoxygenation activate Src family tyrosine kinases and p21ras in cultured rat cardiac myocytes. Biochem Biophys Res Commun. 1996; 226:530–5.
59. Satoh K, Nigro P, Zeidan A, Soe NN, Jaffre F, Oikawa M, et al. Cyclophilin A promotes cardiac hypertrophy in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2011; 31:1116–23.

60. Fratelli M, Demol H, Puype M, Casagrande S, Eberini I, Salmona M, et al. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc Natl Acad Sci U S A. 2002; 99:3505–10.

61. Ghezzi P, Casagrande S, Massignan T, Basso M, Bellacchio E, Mollica L, et al. Redox regulation of cyclophilin A by glutathionylation. Proteomics. 2006; 6:817–25.

62. Massignan T, Casoni F, Basso M, Stefanazzi P, Biasini E, Tortarolo M, et al. Proteomic analysis of spinal cord of presymptomatic amyotrophic lateral sclerosis G93A SOD1 mouse. Biochem Biophys Res Commun. 2007; 353:719–25.

63. Lammers M, Neumann H, Chin JW, James LC. Acetylation regulates cyclophilin A catalysis, immunosuppression and HIV isomerization. Nat Chem Biol. 2010; 6:331–7.

64. Bryant SR, Bjercke RJ, Erichsen DA, Rege A, Lindner V. Vascular remodeling in response to altered blood flow is mediated by fibroblast growth factor-2. Circ Res. 1999; 84:323–8.

65. Chiang HY, Korshunov VA, Serour A, Shi F, Sottile J. Fibronectin is an important regulator of flow-induced vascular remodeling. Arterioscler Thromb Vasc Biol. 2009; 29:1074–9.

66. Acevedo L, Yu J, Erdjument-Bromage H, Miao RQ, Kim JE, Fulton D, et al. A new role for Nogo as a regulator of vascular remodeling. Nat Med. 2004; 10:382–8.

67. Carmeliet P, Moons L, Herbert JM, Crawley J, Lupu F, Lijnen R, et al. Urokinase but not tissue plasminogen activator mediates arterial neointima formation in mice. Circ Res. 1997; 81:829–39.

68. Filippov S, Koenig GC, Chun TH, Hotary KB, Ota I, Bugge TH, et al. MT1-matrix metalloproteinase directs arterial wall invasion and neointima formation by vascular smooth muscle cells. J Exp Med. 2005; 202:663–71.

69. Hassan GS, Jasmin JF, Schubert W, Frank PG, Lisanti MP. Caveolin-1 deficiency stimulates neointima formation during vascular injury. Biochemistry. 2004; 43:8312–21.

70. Korshunov VA, Berk BC. Flow-induced vascular remodeling in the mouse: a model for carotid intima-media thickening. Arterioscler Thromb Vasc Biol. 2003; 23:2185–91.
71. Korshunov VA, Berk BC. Strain-dependent vascular remodeling: the "Glagov phenomenon" is genetically determined. Circulation. 2004; 110:220–6.
72. Ruef J, Hu ZY, Yin LY, Wu Y, Hanson SR, Kelly AB, et al. Induction of vascular endothelial growth factor in balloon-injured baboon arteries. A novel role for reactive oxygen species in atherosclerosis. Circ Res. 1997; 81:24–33.
73. Ruef J, Liu SQ, Bode C, Tocchi M, Srivastava S, Runge MS, et al. Involvement of aldose reductase in vascular smooth muscle cell growth and lesion formation after arterial injury. Arterioscler Thromb Vasc Biol. 2000; 20:1745–52.

74. Leite PF, Danilovic A, Moriel P, Dantas K, Marklund S, Dantas AP, et al. Sustained decrease in superoxide dismutase activity underlies constrictive remodeling after balloon injury in rabbits. Arterioscler Thromb Vasc Biol. 2003; 23:2197–202.

75. Hsieh HJ, Cheng CC, Wu ST, Chiu JJ, Wung BS, Wang DL. Increase of reactive oxygen species (ROS) in endothelial cells by shear flow and involvement of ROS in shear-induced c-fos expression. J Cell Physiol. 1998; 175:156–62.

76. Castier Y, Brandes RP, Leseche G, Tedgui A, Lehoux S. p47phox-dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ Res. 2005; 97:533–40.

77. Castier Y, Ramkhelawon B, Riou S, Tedgui A, Lehoux S. Role of NF-kappaB in flow-induced vascular remodeling. Antioxid Redox Signal. 2009; 11:1641–9.
78. Menshikov M, Plekhanova O, Cai H, Chalupsky K.
Parfyonova Y., Bashtrikov P, et al. Urokinase plasminogen activator stimulates vascular smooth muscle cell proliferation via redox-dependent pathways. Arterioscler Thromb Vasc Biol. 2006. 26:801–7.
79. Seki Y, Kai H, Shibata R, Nagata T, Yasukawa H, Yoshimura A, et al. Role of the JAK/STAT pathway in rat carotid artery remodeling after vascular injury. Circ Res. 2000; 87:12–8.

80. Lambert CM, Roy M, Meloche J, Robitaille GA, Agharazii M, Richard DE, et al. Tumor necrosis factor inhibitors as novel therapeutic tools for vascular remodeling diseases. Am J Physiol Heart Circ Physiol. 2010; 299:H995–1001.

81. El Mabrouk M, Touyz RM, Schiffrin EL. Differential ANG II-induced growth activation pathways in mesenteric artery smooth muscle cells from SHR. Am J Physiol Heart Circ Physiol. 2001; 281:H30–9.

83. Berk BC. Redox signals that regulate the vascular response to injury. Thromb Haemost. 1999; 82:810–7.

84. Touyz RM, Wu XH, He G, Park JB, Chen X, Vacher J, et al. Role of c-Src in the regulation of vascular contraction and Ca2+ signaling by angiotensin II in human vascular smooth muscle cells. J Hypertens. 2001; 19:441–9.

85. Ishida M, Ishida T, Thomas SM, Berk BC. Activation of extracellular signal-regulated kinases (ERK1/2) by angiotensin II is dependent on c-Src in vascular smooth muscle cells. Circ Res. 1998; 82:7–12.

86. Saito Y, Haendeler J, Hojo Y, Yamamoto K, Berk BC. Receptor heterodimerization: essential mechanism for platelet-derived growth factor-induced epidermal growth factor receptor transactivation. Mol Cell Biol. 2001; 21:6387–94.

87. Yang H, Li M, Chai H, Yan S, Lin P, Lumsden AB, et al. Effects of cyclophilin A on cell proliferation and gene expressions in human vascular smooth muscle cells and endothelial cells. J Surg Res. 2005; 123:312–9.
88. Yang Y, Lu N, Zhou J, Chen ZN, Zhu P. Cyclophilin A up-regulates MMP-9 expression and adhesion of mono-cytes/macrophages via CD147 signalling pathway in rheumatoid arthritis. Rheumatology (Oxford). 2008; 47:1299–310.

89. Yurchenko V, Zybarth G, O’Connor M, Dai WW, Franchin G, Hao T, et al. Active site residues of cyclophilin A are crucial for its signaling activity via CD147. J Biol Chem. 2002; 277:22959–65.

90. Obchoei S, Weakley SM, Wongkham S, Wongkham C, Sawanyawisuth K, Yao Q, et al. Cyclophilin A enhances cell proliferation and tumor growth of liver fluke-associated cholangiocarcinoma. Mol Cancer. 2011; 10:102.

91. Yang H, Chen J, Yang J, Qiao S, Zhao S, Yu L. Cyclophilin A is upregulated in small cell lung cancer and activates ERK1/2 signal. Biochem Biophys Res Commun. 2007; 361:763–7.

92. Li M, Zhai Q, Bharadwaj U, Wang H, Li F, Fisher WE, et al. Cyclophilin A is overexpressed in human pancreatic cancer cells and stimulates cell proliferation through CD147. Cancer. 2006; 106:2284–94.

93. Artus C, Boujrad H, Bouharrour A, Brunelle MN, Hoos S, Yuste VJ, et al. AIF promotes chromatinolysis and caspase-independent programmed necrosis by interacting with histone H2AX. EMBO J. 2010; 29:1585–99.

94. Elbaz B, Valitsky M, Davidov G, Rahamimoff H. Cyclophilin A is involved in functional expression of the Na(+)-Ca(2+) exchanger NCX1. Biochemistry. 2010; 49:7634–42.

96. Xu Q, Leiva MC, Fischkoff SA, Handschumacher RE, Lyttle CR. Leukocyte chemotactic activity of cyclophilin. J Biol Chem. 1992; 267:11968–71.

97. Wang L, Wang CH, Jia JF, Ma XK, Li Y, Zhu HB, et al. Contribution of cyclophilin A to the regulation of inflammatory processes in rheumatoid arthritis. J Clin Immunol. 2010; 30:24–33.

98. Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000; 105:1605–12.

99. Feldman DS, Zamah AM, Pierce KL, Miller WE, Kelly F, Rapacciuolo A, et al. Selective inhibition of hetero-trimeric Gs signaling. Targeting the receptor-G protein interface using a peptide minigene encoding the Galpha(s) carboxyl terminus. J Biol Chem. 2002; 277:28631–40.
100. Alexis JD, Wang N, Che W, Lerner-Marmarosh N, Sahni A, Korshunov VA, et al. Bcr kinase activation by angiotensin II inhibits peroxisome-proliferator-activated receptor gamma transcriptional activity in vascular smooth muscle cells. Circ Res. 2009; 104:69–78.
101. McCormick ML, Gavrila D, Weintraub NL. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2007; 27:461–9.

102. Bruemmer D, Collins AR, Noh G, Wang W, Territo M, Arias-Magallona S, et al. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest. 2003; 112:1318–31.

103. Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, et al. Targeted gene disruption of matrix metal-loproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000; 105:1641–9.

104. Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002; 110:625–32.

105. Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003; 92:827–39.
106. Aartsen WM, Hilgers RH, Schiffers PM, Daemen MJ, De Mey JG, Smits JF. Changes in vascular distensibility during angiotensin-converting enzyme inhibition involve bradykinin type 2 receptors. J Vasc Res. 2004; 41:18–27.

107. Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2009; 29:1458–64.

108. Browatzki M, Larsen D, Pfeiffer CA, Gehrke SG, Schmidt J, Kranzhofer A, et al. Angiotensin II stimulates matrix metalloproteinase secretion in human vascular smooth muscle cells via nuclear factor-kappaB and activator protein 1 in a redox-sensitive manner. J Vasc Res. 2005; 42:415–23.
109. Luchtefeld M, Grote K, Grothusen C, Bley S, Bandlow N, Selle T, et al. Angiotensin II induces MMP-2 in a p47phox-dependent manner. Biochem Biophys Res Commun. 2005; 328:183–8.

110. Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. J Clin Invest. 1996; 98:2572–9.
111. Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006; 6:508–19.

113. Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999; 340:115–26.
114. Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008; 8:802–15.

115. Rahman A, Kefer J, Bando M, Niles WD, Malik AB. E-selectin expression in human endothelial cells by TNF-alpha-induced oxidant generation and NF-kappaB activation. Am J Physiol. 1998; 275:L533–44.
116. Tricot O, Mallat Z, Heymes C, Belmin J, Leseche G, Tedgui A. Relation between endothelial cell apoptosis and blood flow direction in human atherosclerotic plaques. Circulation. 2000; 101:2450–3.

117. Ostergaard L, Simonsen U, Eskildsen-Helmond Y, Vorum H, Uldbjerg N, Honore B, et al. Proteomics reveals lowering oxygen alters cytoskeletal and endoplasmatic stress proteins in human endothelial cells. Proteomics. 2009; 9:4457–67.

118. Nigro P, Satoh K, O’Dell MR, Soe NN, Cui Z, Mohan A, et al. Cyclophilin A is an inflammatory mediator that promotes atherosclerosis in apolipoprotein E-deficient mice. J Exp Med. 2011; 208:53–66.

120. Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007; 292:C82–97.

121. Sadoshima J, Izumo S. Molecular characterization of angiotensin II–induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993; 73:413–23.
122. Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, Ohe T, et al. Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation. 1998; 98:794–9.
123. Akki A, Zhang M, Murdoch C, Brewer A, Shah AM. NADPH oxidase signaling and cardiac myocyte function. J Mol Cell Cardiol. 2009; 47:15–22.

124. Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007; 49:241–8.

125. Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin-angiotensin-aldosterone system. Circulation. 1991; 83:1849–65.
127. Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010; 106:360–8.

128. Prasad A, Stone GW, Holmes DR, Gersh B. Reperfusion injury, microvascular dysfunction, and cardioprotection: the "dark side" of reperfusion. Circulation. 2009; 120:2105–12.
129. Hess ML, Manson NH. Molecular oxygen: friend and foe. The role of the oxygen free radical system in the calcium paradox, the oxygen paradox and ischemia/reperfusion injury. J Mol Cell Cardiol. 1984; 16:969–85.
130. Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004; 61:461–70.

131. Braunersreuther V, Jaquet V. Reactive oxygen species in myocardial reperfusion injury: from physiopathology to therapeutic approaches. Curr Pharm Biotechnol. 2011; Apr 6 [Epub].

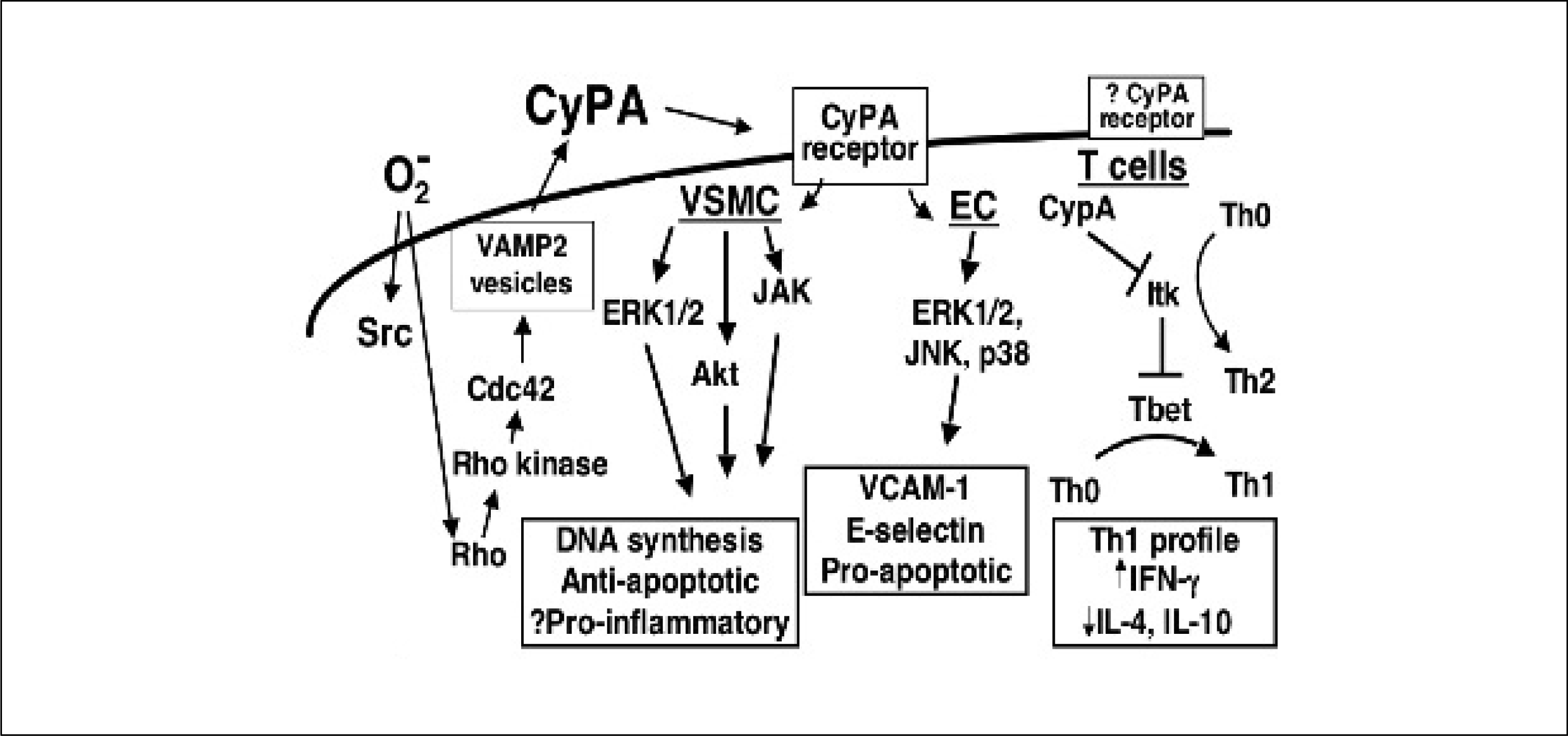
Fig. 1.
Cyclophilin A (CyPA) effects on vascular smooth muscle (VSMC), endothelial cells (EC) and T cells. VCAM-1, vascular cell adhesion molecule-1; IFN, interferon; IL, interleukin.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download