ABSTRACT
Background:
The aim of this study was to compare the antihypertensive effect of S-(-)-amlodipine nicotinate with ramipril in patients with essential hypertension.
Methods:
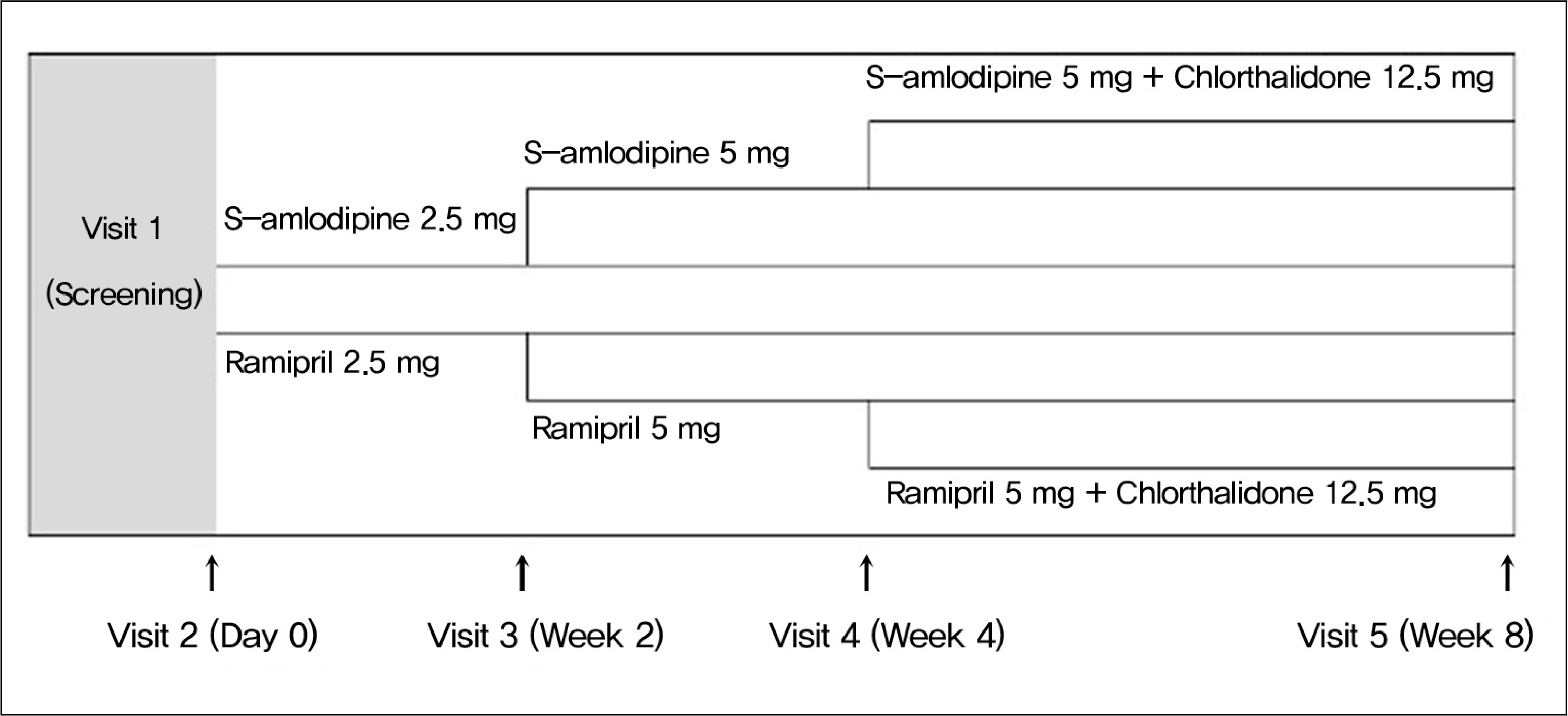
Total 138 patients (54.5 ± 10.5 years, 69 males) were enrolled in this study between 2008 and 2010. Amlodipine 2.5 mg or ramipril 2.5 mg was treated once in a day for 8 weeks. Epidemiologic analysis was performed in intend-to-treat group. Efficacy analysis was performed in the differences of diastolic blood pressure in study groups. Abnormal reactions were divided with severities and drug-relationship.
Results:
The change of diastolic blood pressures were more prominent with -12.7 ± 7.02 mm Hg in amlodipine group, and -9.6 ± 7.38 mm Hg in ramipril group (p = 0.023). The change of systolic blood pressures was higher in amlodipine group with -18.1 ± 7.91 mm Hg, and -14.3 ± 11.96 mm Hg in ramipril group (p = 0.047). Blood pressure normalization rates were 81.3% (48 of 59 patients) in amlodipine group, and 61.4% (35 of 57 patients) in ramipril group (p = 0.017). Abnormal reaction occurred in 5.8% (4 of 68 patients) of amlodipine group and 14.2% (10 of 70 patients) of ramipril group (p = 0.102). The most frequent abnormal reaction was respiratory symptom.
Go to : 
References
1. Whitworth JA, World Health Organization , International Society of Hypertension Writing Group . 2003 World Health Organization (WHO)/ International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003. 21:p. 1983–92.
2. Weber MA. Hypertension treatment and implications of recent cardiovascular outcome trials. J Hypertens Suppl. 2006; 24:S37–44.

3. Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF, et al. Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004-BHS IV. J Hum Hypertens. 2004; 18:139–85.

4. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003; 289:2560–72.

5. Youn JS, Ahn YS, Hwang YJ, Jung HM, Kim WJ, Lee MG, et al. Single center, randomized, single-blind, parallel-group, phase IV clinical trial for the comparison of efficacy and safety between S-(-)-Amlodipine Nicotinate and Ler-canidipine HCl in patients with hypertension. J Korean Soc Hypertens. 2010; 16:18–30.
6. Bae JH, Jun JE, Lee MM, Kim CH, Hyon MS, Choe KH, et al. Double-blind, randomized, multi-center trial for the comparison of efficacy and safety between S-amlodipine besylate and Amlodipine besylate in patients with hypertension. J Korean Soc Hypertens. 2008; 14:28–36.
7. Omvik P, Herland OB, Thaulow E, Eide I, Midha R, Turner RR. Evaluation and quality-of-life assessment of amlodipine and enalapril in patients with hypertension. J Hum Hypertens. 1995; 9(Suppl 1):S17–24.
8. Karch FE, Pordy R, Benz JR, Carr A, Lunde NM, Marbury T, et al. Comparative efficacy and tolerability of two long-acting calcium antagonists, mibefradil and amlodipine, in essential hypertension. Mibefradil Hypertension Study Group. Clin Ther. 1997; 19:1368–78.
9. Park JY, Kim KA, Park PW, Lee OJ, Ryu JH, Lee GH, et al. Pharmacokinetic and pharmacodynamic characteristics of a new S-amlodipine formulation in healthy Korean male subjects: a randomized, open-label, two-period, comparative, crossover study. Clin Ther. 2006; 28:1837–47.

10. Perticone F, Pugliese F, Ceravolo R, Mattioli PL. Amlodipine versus ramipril in the treatment of mild to moderate hypertension: evaluation by 24-hour ambulatory blood pressure monitoring. Cardiology. 1994; 85:36–46.

11. Lund-Johansen P, Stranden E, Helberg S, Wessel-Aas T, Risberg K, Ronnevik PK, et al. Quantification of leg oedema in postmenopausal hypertensive patients treated with lercanidipine or amlodipine. J Hypertens. 2003; 21:1003–10.

12. Kaplan NM. The CARE Study: a postmarketing evaluation of ramipril in 11,100 patients. The Clinical Altace Real-World Efficacy (CARE) Investigators. Clin Ther. 1996; 18:658–70.
13. ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002; 288:2981–97.
14. Agabiti-Rosei E, Ambrosioni E, Dal Palu C, Muiesan ML, Zanchetti A. ACE inhibitor ramipril is more effective than the beta-blocker atenolol in reducing left ventricular mass in hypertension. Results of the RACE (ramipril cardioprotective evaluation) study on behalf of the RACE study group. J Hypertens. 1995; 13:1325–34.
15. Fuster RG, Montero Argudo JA, Albarova OG, Hornero Sos F, Canovas Lopez S, Bueno Codoner M, et al. Left ventricular mass index as a prognostic factor in patients with severe aortic stenosis and ventricular dysfunction. Interact Cardiovasc Thorac Surg. 2005; 4:260–6.

16. Kim EJ, Yoo JY, Cheon WS, Han SW, Choi YJ, Ryu KH, et al. Coronary artery size in Korean: normal value and its determinants. Korean Circ J. 2005; 35:115–22.

17. Julius S, Kjeldsen SE, Brunner H, Hansson L, Platt F, Ekman S, et al. VALUE trial: Long-term blood pressure trends in 13,449 patients with hypertension and high cardiovascular risk. Am J Hypertens. 2003; 16:544–8.

18. Kidney Disease Outcomes Quality Initiative (K/DOQI) . K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004; 43:S1–290.
19. Kim KH, Jeong MH, Cho SH, Moon JY, Hong YJ, Park HW, et al. Clinical effects of calcium channel blocker and angiotensin converting enzyme inhibitor on endothelial function and arterial stiffness in patients with angina pectoris. J Korean Med Sci. 2009; 24:223–31.

Go to : 
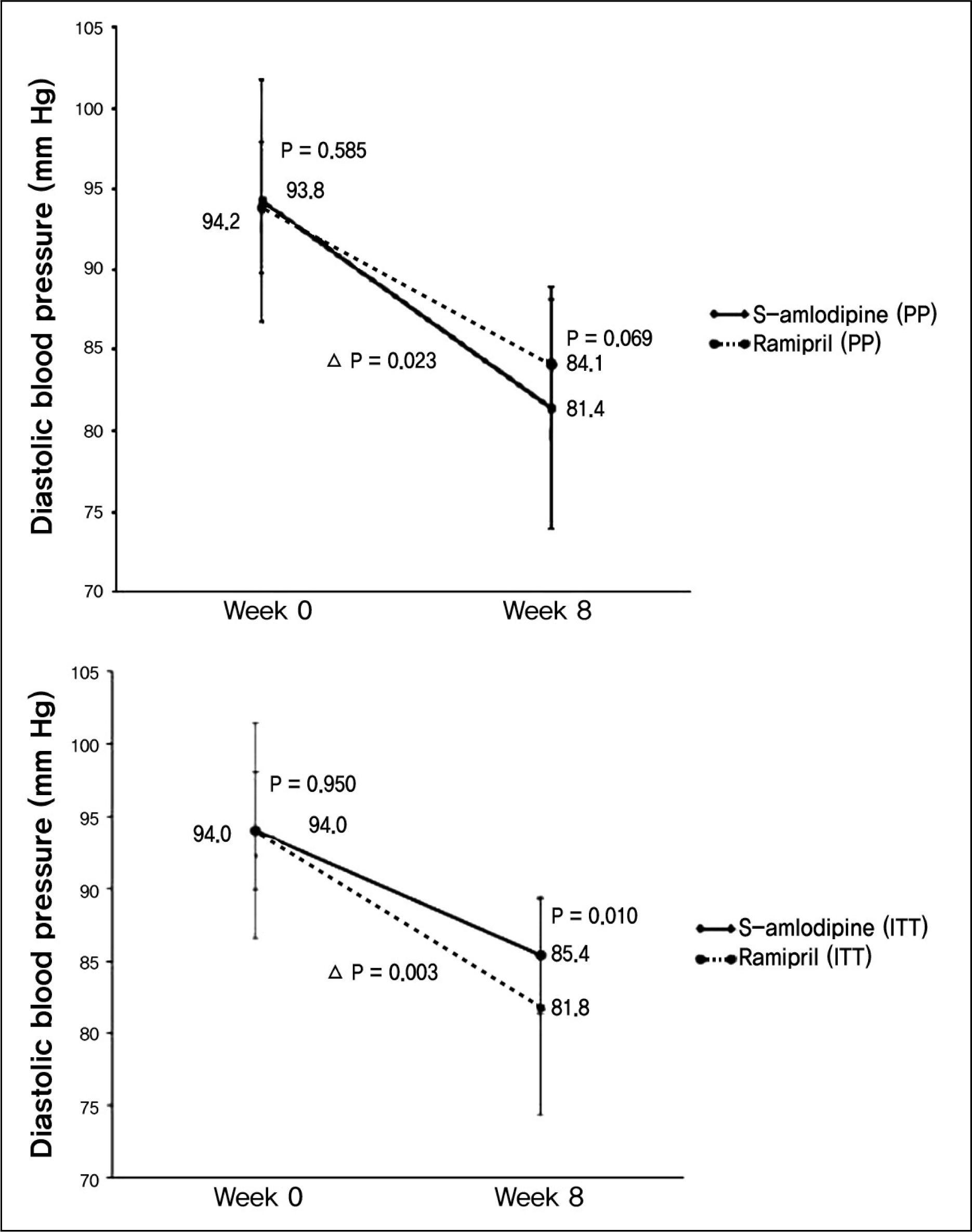 | Fig. 2.Efficacy evaluation with diastolic blood pressure decrement comparing Week 0 with Week 8 in per-protocol and intent-to-treat group. |
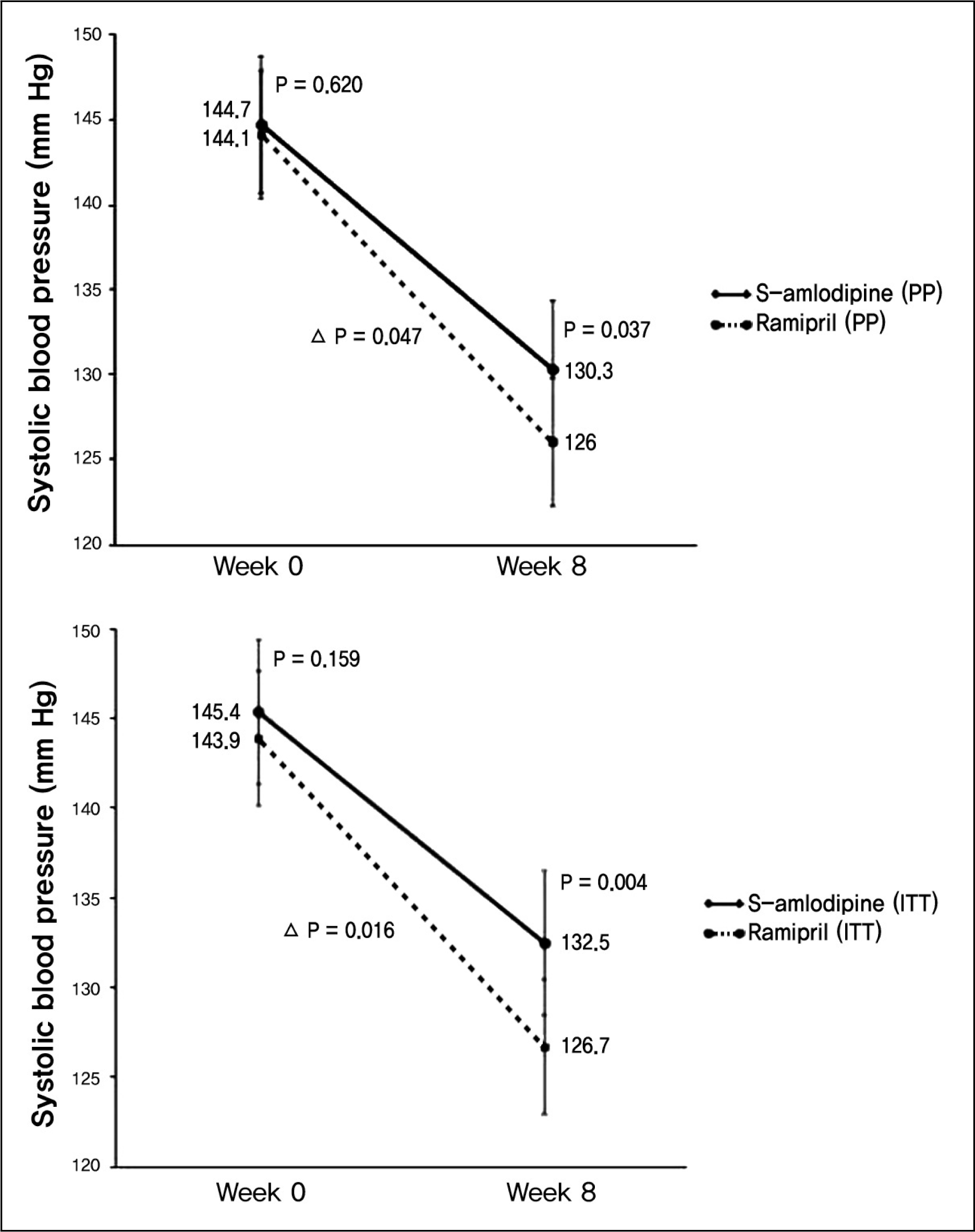 | Fig. 3.Efficacy evaluation with systolic blood pressure decrement comparing Week 0 with Week 8 in per-protocol and intent-to-treat group. |
Table 1.
Grades of blood pressure reduction according to guidance for the clinical trials evaluation for hypertension
Table 2.
Baseline clinical characteristics in S-amlodipine and Ramipril groups
Table 3.
Efficacy evaluation with blood pressure decrement
Table 4.
Efficacy evaluation in per-protocol group
Table 5.
Classified distribution of abnormal reaction




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download