ABSTRACT
Although evidence-based medicine (EBM) has changed medical therapy from experience- or experiment-based medicine to the modern science-based medicine, nowadays it seems to be used as a tool of advertizing strategy of industries. Clinical trial is a fundamental component of EBM and it costs very much and are mostly conducted by the financial support of pharmaceutical industries at a tremendous expense. If anticipated results for the sponsor were not shown in the trial, a “spin” has been used to mislead readers as if positive results for test drugs or devices were obtained by emphasizing subgroup analysis, or searching favorable endpoint by post-hoc analysis. Clinical trials on angiotensin receptor blocker (ARB) are continuing succession of defeat all over world except two Japanese clinical trials, JIKEI HEART and KYOTO HEART studies. However, in both trials, subjective soft endpoints, such as angina pectoris or congestive heart failure were used despite of prospective randomized, open labeled, blinded endpoint design. In the era of advertizing-based medicine, It is important to read clinical trial data with critical view points.
References
1. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999. 353:p. 9–13.
2. Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991; 324:781–8.
3. Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991; 265:3255–64.
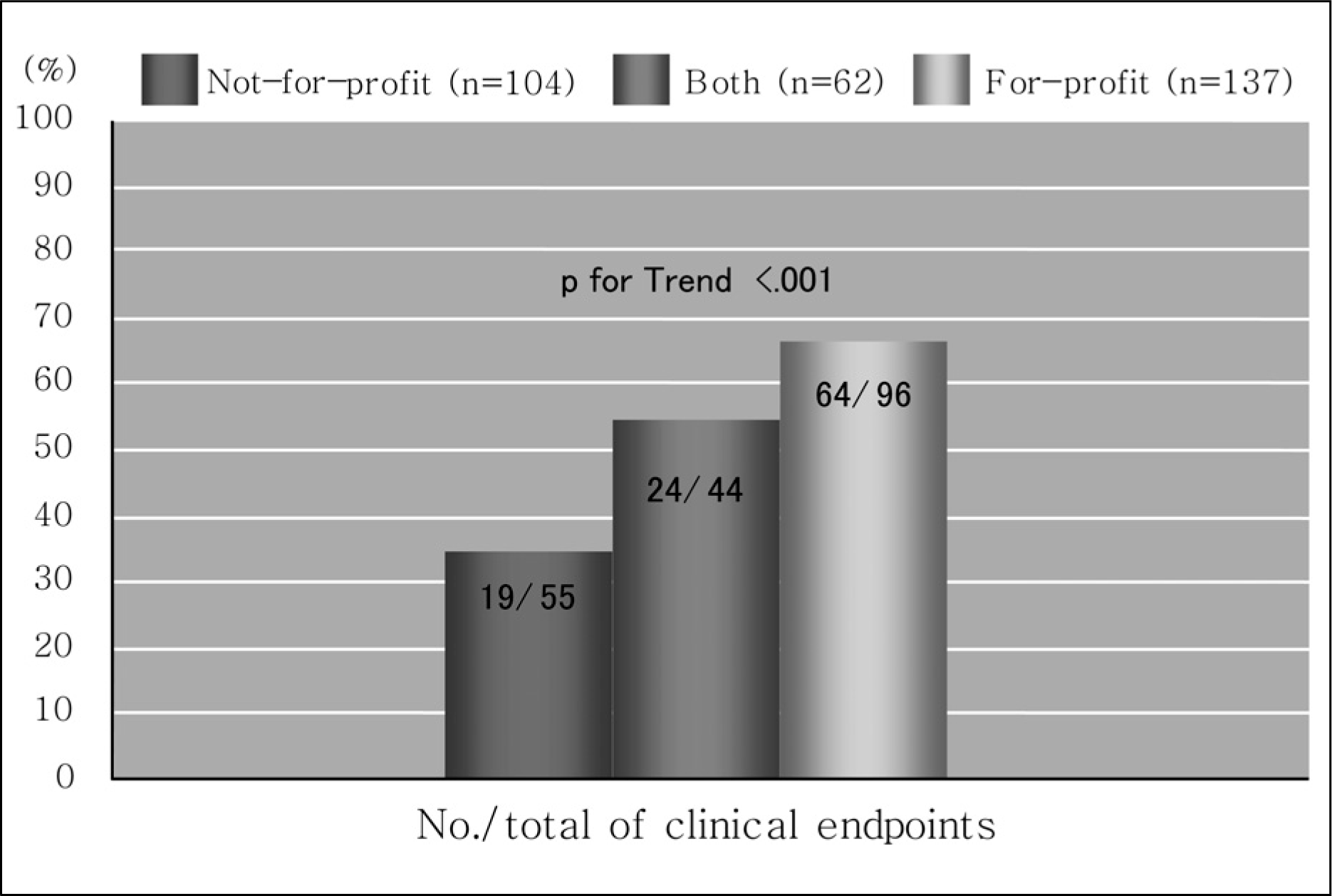
4. Ridker PM, Torres J. Reported outcomes in major cardiovascular clinical trials funded by for-profit and not-for-profit organizations: 2000-2005. JAMA. 2006; 295:2270–4.

5. Packer M, O’Connor CM, Ghali JK, Pressler ML, Carson PE, Belkin RN, et al. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. Prospective Randomized Amlodipine Survival Evaluation Study Group. N Engl J Med. 1996; 335:1107–14.
6. Black HR, Elliott WJ, Grandits G, Grambsch P, Lucente T, White WB, et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003; 289:2073–82.

7. Boutron I, Dutton S, Ravaud P, Altman DG. Reporting and interpretation of randomized controlled trials with statistically nonsignificant results for primary outcomes. JAMA. 2010; 303:2058–64.

8. Thackray S, Witte K, Clark AL, Cleland JG. Clinical trials update: OPTIME-CHF, PRAISE-2, ALL-HAT. Eur J Heart Fail. 2000; 2:209–12.

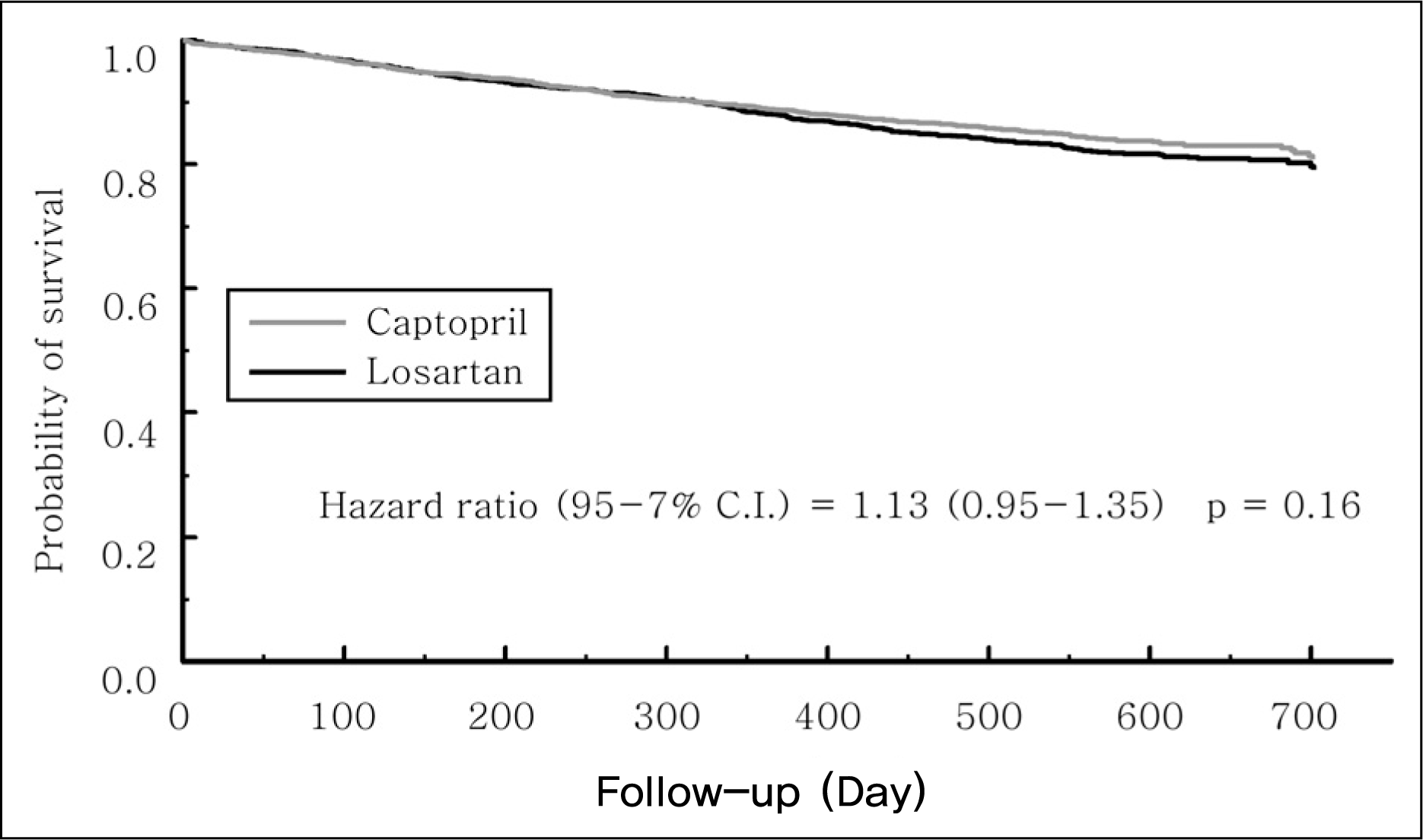
9. Pitt B, Segal R, Martinez FA, Meurers G, Cowley AJ, Thomas I, et al. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE). Lancet. 1997; 349:747–52.

10. Pitt B, Poole-Wilson PA, Segal R, Martinez FA, Dickstein K, Camm AJ, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: randomised trial–the Losartan Heart Failure Survival Study ELITE II. Lancet. 2000; 355:1582–7.

11. Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004; 363:2022–31.

12. Weber MA, Julius S, Kjeldsen SE, Brunner HR, Ekman S, Hansson L, et al. Blood pressure dependent and independent effects of antihypertensive treatment on clinical events in the VALUE Trial. Lancet. 2004; 363:2049–51.

13. Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008; 358:1547–59.

14. Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, Copland I, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008; 372:1174–83.
15. Disertori M, Latini R, Barlera S, Franzosi MG, Staszewsky L, Maggioni AP, et al. Valsartan for prevention of recurrent atrial fibrillation. N Engl J Med. 2009; 360:1606–17.
16. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. 2008; 359:2456–67.

17. Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008; 359:1225–37.

18. Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009; 361:40–51.

19. Holman RR, Haffner SM, McMurray JJ, Bethel MA, Holzhauer B, Hua TA, et al. Effect of nateglinide on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010; 362:1463–76.

20. Mochizuki S, Dahlof B, Shimizu M, Ikewaki K, Yoshikawa M, Taniguchi I, et al. Valsartan in a Japanese population with hypertension and other cardiovascular disease (Jikei Heart Study): a randomised, open-label, blinded endpoint morbidity-mortality study. Lancet. 2007; 369:1431–9.
21. Sawada T, Yamada H, Dahlof B, Matsubara H. Effects of valsartan on morbidity and mortality in uncontrolled hypertensive patients with high cardiovascular risks: KYOTO HEART Study. Eur Heart J. 2009; 30:2461–9.

22. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002; 288:2981–97.
23. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). ALLHAT Collaborative Research Group. JAMA. 2000; 283:1967–75.
24. Kostis JB, Wilson AC, Freudenberger RS, Cosgrove NM.
Pressel SL., Davis BR, et al. Long-term effect of diuretic-based therapy on fatal outcomes in subjects with isolated systolic hypertension with and without diabetes. Am J Cardiol. 2005. 95:29–35.
25. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998. 352:p. 837–53.
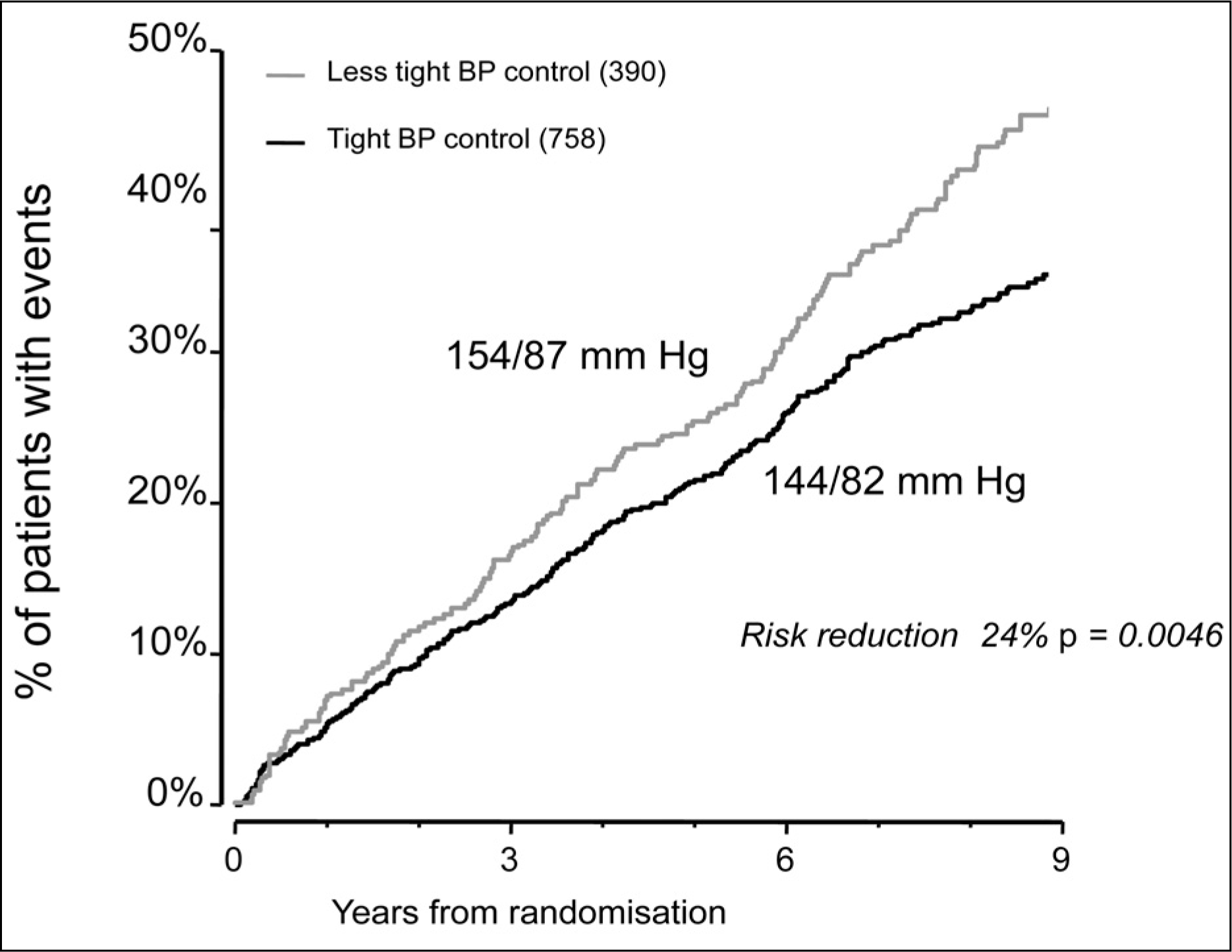
26. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998. 317:p. 703–13.
27. Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000; 342:145–53.
Fig. 3.
VThe Valsartan Antihypertensive Long-term Use Evaluation: hazard ratio of primary and secondary endpoints.10)
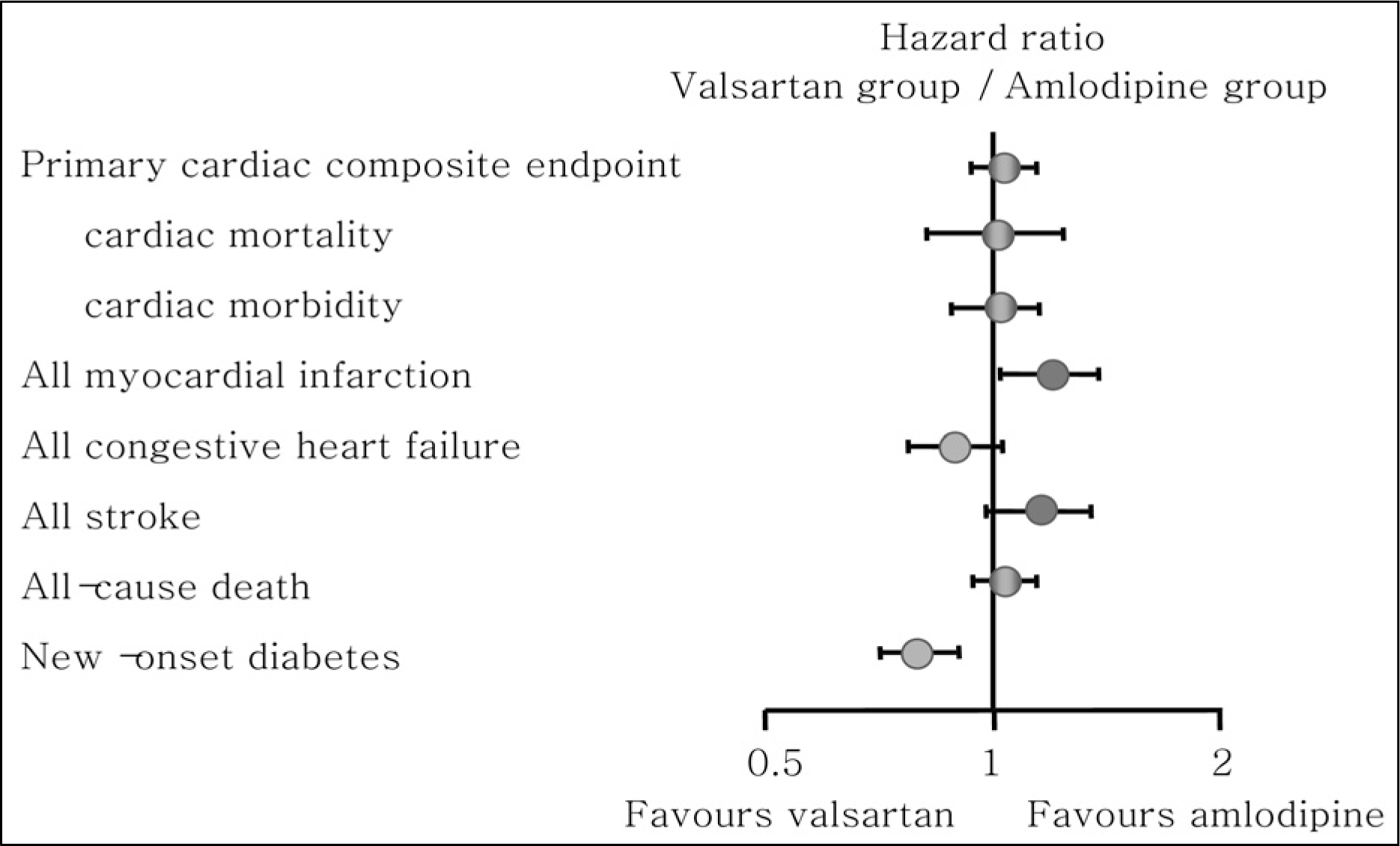
Fig. 4.
Hazard ratios (HR) for major endpoints in patients on valsartan or amlodipine based therapies. Events occurring after a baseline transiocated to the 6 months point of the trial (after adjustment designed to achieve BP control) in 5006 treament cohort pairs matched by SBP, age, sex and the presence or absence of prior coronary disease, stroke and diabetes.12) CI, confidence interval.
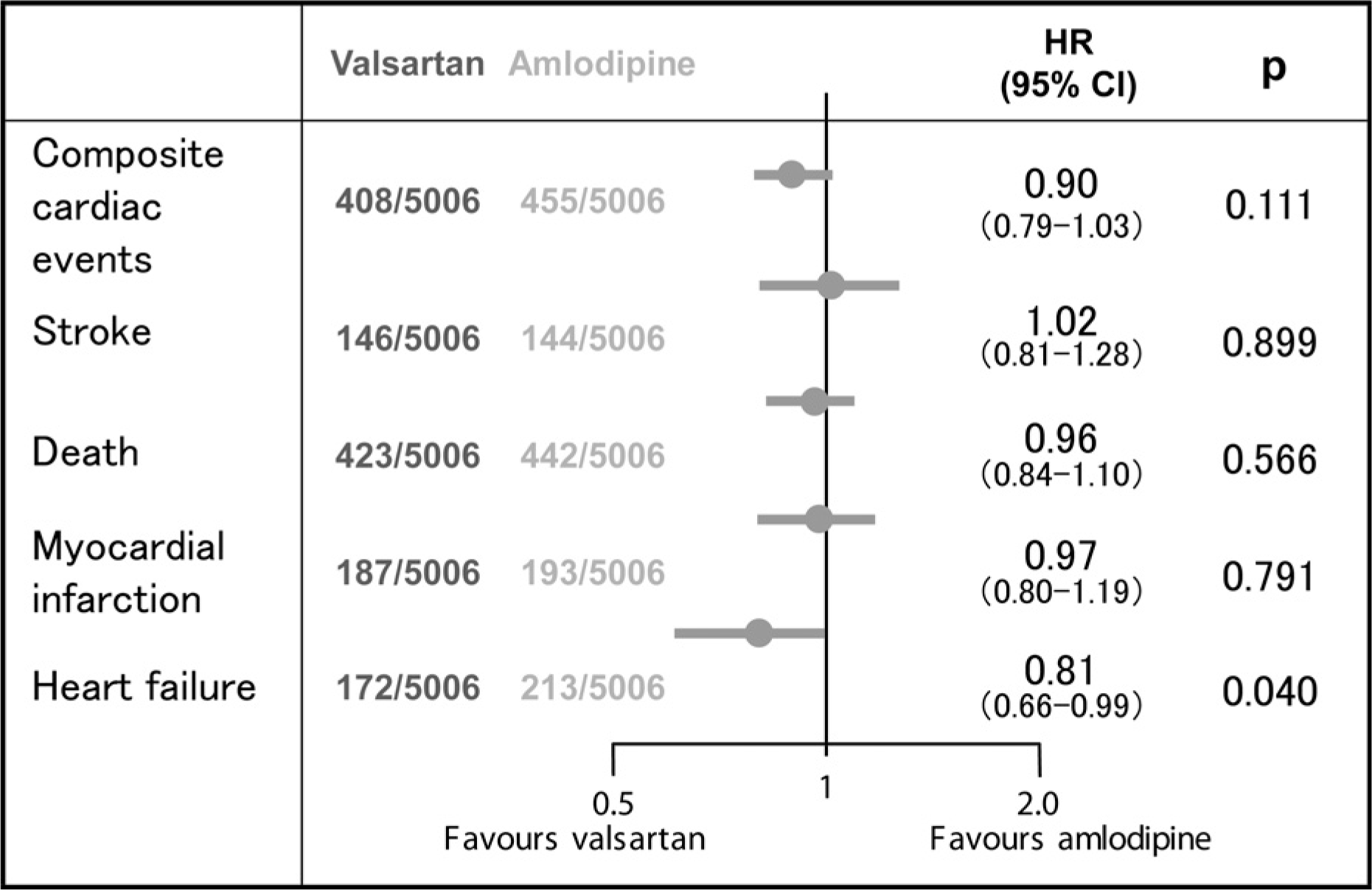
Fig. 5.
KYOTO HEART study. Hazard ratio and 95%confidence intervals adjusted for sex, age, diabetes, smoking, dyslipidemia, and concomitant antihypertensive treatment.21) ARB, angiotensin receptor blocker.
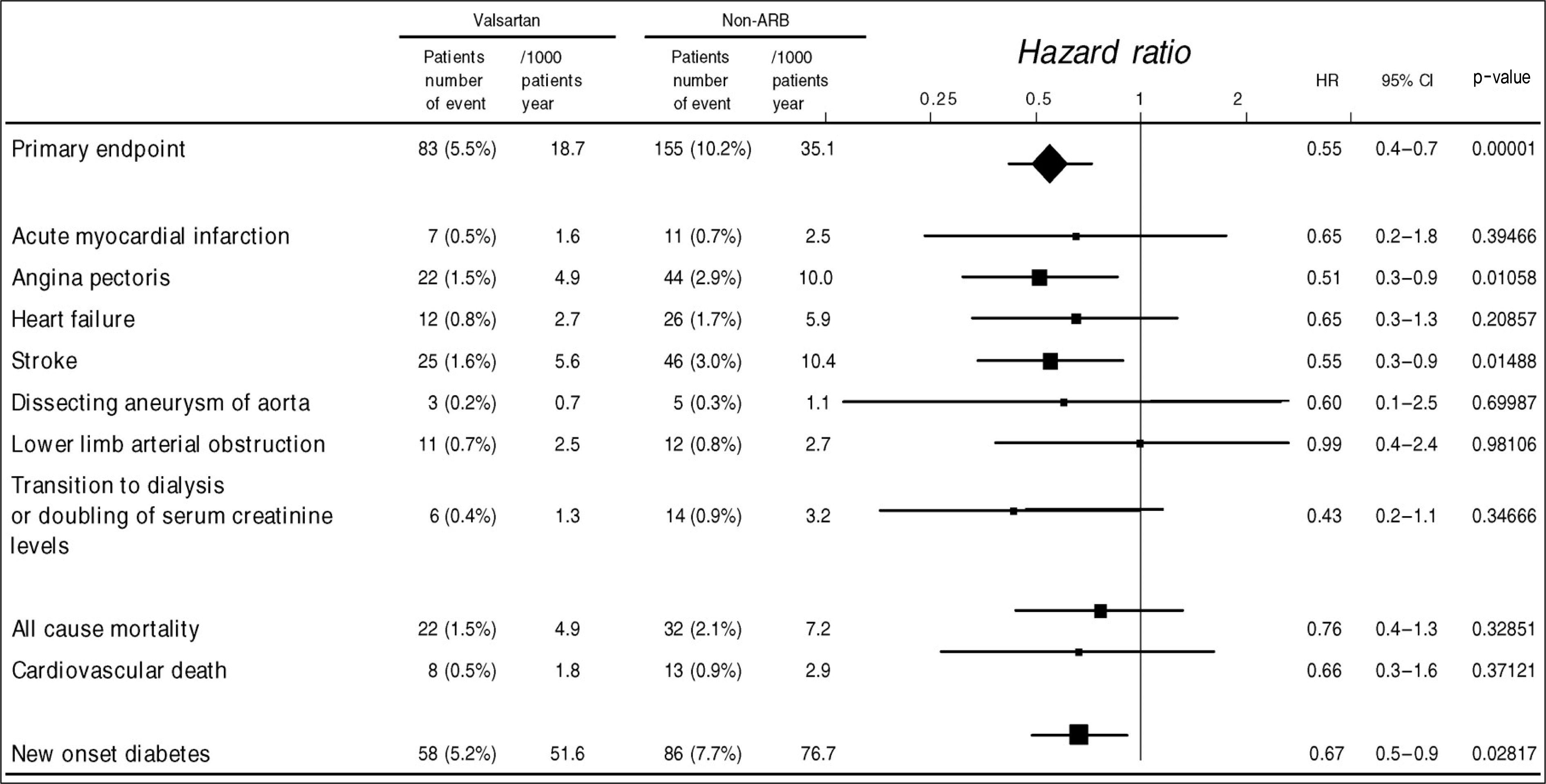
Fig. 7.
ACE inhibitors in stable coronary artery disease. Comparison of outcomes in the PEACE and HOPE trials.28) ACE, angiotensin converting enzyme; HOPE, Heart Outcomes Prevention Evaluation; PEACE, Prevention of Events with Angiotensin-Converting Enzyme inhibition.
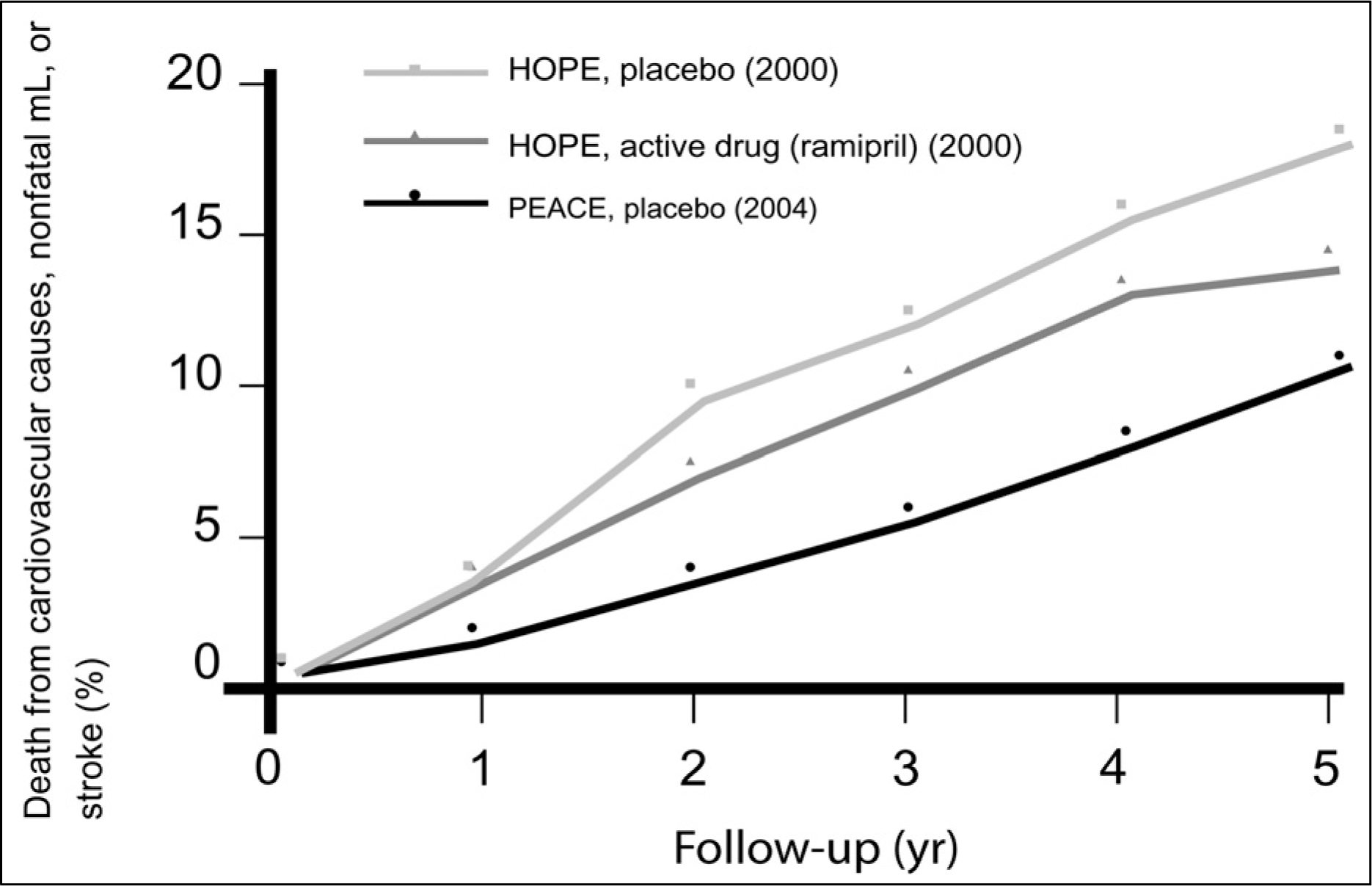
Table 1.
Changing therapy for coronary disease
| Concomitant therapy (%) | HOPE (2000) | PEACE (2004) | ONTARGET (2008) | TRANSCEND (2008) |
|---|---|---|---|---|
| Statin | 29 | 70 | 62 | 55 |
| Antiplatelets | 76 | 90 | 76 | 80 |
| Beta blocker | 39 | 60 | 58 | 58 |
| Coronary revascularization | 40 | 72 | 51 | 46 |
HOPE, Heart Outcomes Prevention Evaluation; PEACE, Prevention of Events with Angiotensin-Converting Enzyme inhibition; ONTARGET, The ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial; TRANSCEND, Telmisartan Randomized Assessment Study in ACE Intolerant Subjects with Cardiovascular Disease.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download