Introduction
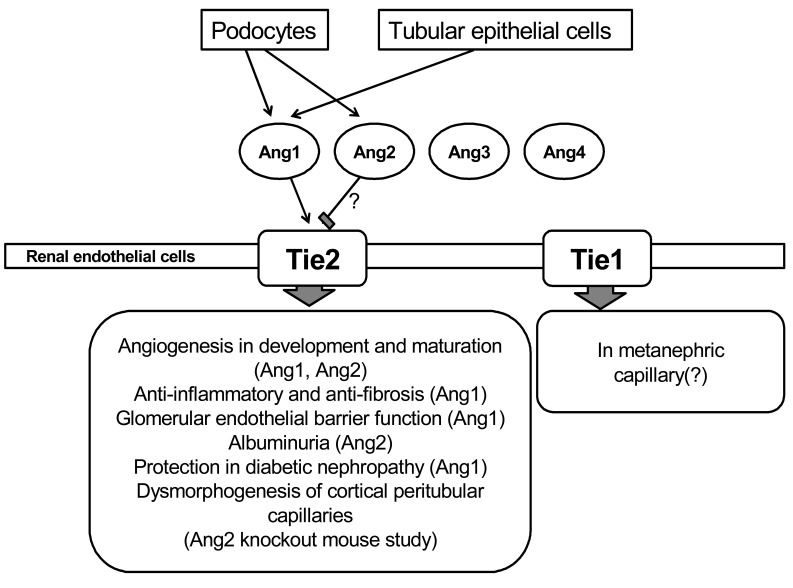
Angiopoietins are a family of growth factors which act on the Tie receptor on endothelial cells. The angiopoietin family consists of 4 angiopoietins, i.e., angiopoietin-1 (Ang1), angiopoietin-2 (Ang2), angiopoietin-3 (Ang3), and angiopoietin-4 (Ang4)1-3). Ang1 is known as an angiogenic factor4), however, the function of Ang2, Ang3 and Ang4 is not well known. Angiopoietins' receptor system consists of two receptor tyrosine kinases, Tie1 and Tie2. The Tie receptors are exclusively expressed by endothelial cells and hematopoietic cells5). It has been also reported that Tie2 is also expressed in macrophages, eosinophils, and hematopoietic stem cells5, 6). Although Tie1 and Tie2 share a similar structure consisting of an extracellular domain with 33% similarity and an intracellular tyrosine kinase domain with 76% similarity, the exact role of Tie1 is not well known 7). Ang1 and Ang2 bind to the same site in Tie2 with similar affinities. Ang1 is a widely expressed ligand for the Tie2 tyrosine kinase receptor that is expressed on endothelial cells, and it regulates vascular growth, development, maturation, and permeability8, 9). Ang1-induced Tie2 phosphorylation in endothelial cells can activate the protein kinase B/Akt pathway that conducts endothelial survival10-12). Although Ang1 is known as an angiogenic factor, it has been also demonstrated that Ang1 has an anti-inflammatory effect13, 14). Although the exact role of Ang2 is not well known, it has been reported that Ang2 functions as an antagonist ligand of Tie215). Ang1 has potential therapeutic applications in inducing angiogenesis, enhancing endothelial cells survival, and preventing vascular leakage12, 16-20) (Fig. 1).
In this section, I focus on the therapeutic effect of Ang1 in kidney disease
Angiopoietin-1 and Tie2 expression in kidney
Ang1 is also associated with physiologic or pathophysiologic conditions in the kidney21). In kidney development, Tie2 is detected in capillaries of the nephrogenic cortex, glomerular tufts, cortical interstitium, and medulla including vessels in the vasa recta22, 23). Thus, Ang1 and Tie2 play roles in the maturation of glomeruli and the vasa rectae. Ang1 protein is detected in podocytes in normal glomeruli and was highly expressed in podocyte foot processes24). Tie2 has been demonstrated on glomerular capillary endothelial cells and peritubular capillary endothelial cells23). Thus, circulating Ang1 may have an effect on renal Tie2 in the endothelial cell and renal Ang1 may also have paracrine effect on renal Tie225, 26).
Disturbance of endothelial integrity in the kidney may cause a reduction in glomerular filtration rate or proteinuria. Ang1 in the glomerulus or renal epithelial cells may have a role in maintenance of the glomerular or peritubular endothelium in the kidney. In pathophysiologic conditions, folic acid-induced nephropathy is associated with increased Ang1 protein expression in renal epithelia and arteries27). It was also reported that loss of glomerular capillaries during the course of anti-glomerular basement membrane glomerulonephritis in mice was temporally associated with decreases in endothelial survival molecules Ang128). Therefore, Ang1 may have an important role in maintenance of normal kidney development of the glomerular or peritubular endothelium and various kidney disease conditions.
Cartilage oligomeric matrix protein-angiopoietin-1 in renal fibrosis
Most forms of chronic kidney disease finally progress to interstitial fibrosis. The severity of tubulointerstitial changes is the best indicator of the progression of renal dysfunction29, 30). Renal tubulointerstitial fibrosis is an important feature in unilateral ureteral obstruction (UUO). In humans, chronic and acute ureteral obstruction can occur in various clinical situations such as ureteral stone or ureteral carcinoma. Injury to the renal microvasculature and inflammatory condition may be major factors in the process of kidney disease31). In particular, injury to the peritubular capillary endothelium of the kidney may be a factor in tubulointerstitial disease32-34). It has been reported that impaired angiogenesis may occur in the diseased kidney and can contribute to renal scarring33). Renal ischemia that is caused by vascular obliteration can be a major contributor to renal scarring. Therefore, endothelial cells play an important role in renal disease progression in the unilateral ureteral obstructed kidney. Because renal microvasculature injury involves a critical process in renal fibrosis, a growth factor or cytokine with an endothelial protective or angiogenic effect may have a protective role in renal fibrosis in the UUO model35-37).
Recently, a variant of Ang1, cartilage oligomeric matrix protein (COMP)-Ang1 was recently developed. COMP-Ang1 is soluble and more potent than native Ang1 in phosphorylating Tie2 and signaling via Akt in primary cultured endothelial cells13, 38). Thus, I evaluated the protective effect of COMP-Ang1 in the UUO-induced renal fibrosis model39).
In the UUO model, COMP-Ang1 preserved the renal platelet-endothelial cell adhesion molecule-1- and Tie2-positive endothelial cell. Morphologic examination demonstrated less tubular injury and renal interstitial fibrosis in mice that received COMP-Ang1 than in vehicle-treated mice. Type I collagen and myofibroblast accumulation were significantly decreased by COMP-Ang1 treatment. COMP-Ang1 stimulated Tie2 and Akt phosphorylation in ureteral obstructed kidneys. Renal surface microvasculature and renal blood flow were higher after treatment with COMP-Ang1 than in vehicle-treated mice. COMP-Ang1 treatment suppressed monocyte/macrophage infiltration, tissue levels of transforming growth factor β1 (TGF-β1), and Smad 2/3 phosphorylation and increased Smad 7 in the obstructed kidney. These results suggest that COMP-Ang1 treatment can prevent the progression of renal fibrosis in UUO.
Cartilage oligomeric matrix protein-angiopoietin-1 in diabetic nephropathy model
Inflammatory processes have been recently seen as the important factor in the pathogenesis of diabetic nephropathy. In this study, db/db mice were treated with adenovirus expressing either COMP-Ang1 or LacZ40). To evaluate histology, inflammatory, metabolic, and fibrotic parameters and signalling pathways, we used diabetic nephropathy model.
COMP-Ang1 reduced albuminuria in 24 hour urine analysis and decreased mesangial expansion, thickening of the glomerular basement membrane, and podocyte foot process broadening and effacement in histologic examination. COMP-Ang1 decreased both kidney expression of the adhesion molecule and the number of f4/80-positive macrophage infiltration in diabetic db/db mice. In addition, COMP-Ang1 significantly decreased fasting blood glucose level, epididymal fat weight to body weight ratio, and epididymal adipocyte size in diabetic db/db mice. COMP-Ang1 also ameliorated the fibrotic process in the kidney of diabetic db/db mice through its anti-inflammatory and metabolic effects. COMP-Ang1 suppressed renal levels of TGF-β1, α-smooth muscle actin, fibronectin, as well as Smad 2/3, but increased Smad 7. In human umbilical vein endothelial cells, COMP-Ang1 treatment decreased high glucose-induced nuclear factor-κB (NF-κB) activation. COMP-Ang1 mediated inhibition of increased NF-κB-DNA binding activity in nuclear extracts from human umbilical vein endothelial cells grown in high glucose was significantly blocked by soluble Tie2 receptor-Fc. The side effect of COMP-Ang1 is not well known. However, there are several conditions associated with COMP-Ang1 such as slight increased redness in the nose and increased vascular density in the trachea. These results suggest that COMP-Ang1 ameliorated the fibrotic processes in the kidney of diabetic db/db mice through its anti-inflammatory or metabolic effects.
Cartilage oligomeric matrix protein-angiopoietin-1 in cyclosporine-induced renal injury
In cyclosporine (CsA)-induced renal injury, COMP-Ang1 significantly decreased CsA-induced tubular damage and tubulointerstitial fibrosis in histologic examination. COMP-Ang1 also reduced the number of macrophage infiltration and expression of adhesion molecules in the CsA-induced renal injury model. COMP-Ang1 administration decreased CsA-induced increase of TGF-β1 and Smad 2/3 in kidneys, while increasing Smad 7 levels. Laser-Doppler sonographic findings and endothelial factor VIII staining revealed that COMP-Ang1 had a protective effect on peritubular vasculature and intrarenal hemodynamic alteration in CsA-induced renal injury.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download