Abstract
This study inquired the relationship between serum N-terminal pro-brain natriuretic peptide (NT-proBNP) levels and left ventricular (LV) dysfunction and extracellular water in continuous ambulatory peritoneal dialysis (CAPD) patients. We conducted a cross-sectional study of 30 CAPD patients. Each patient was admitted to the department of internal medicine, Chosun University Hospital between February and October, 2006. Echocardiography was performed using HDI 5000, allowing M-mode, two-dimensional measurement. A multifrequency bioimpedance analyzer was used; extracellular water was calculated as a percentage of total body water and was understood as the index of volume load of CAPD patients. The mean age was 47±12 years. Underlying causes of renal failure were 14 with diabetes mellitus, 7 with hypertension, and 9 with chronic glomerulonephritis. The mean serum NT-proBNP level was 14236.56 (83-35,000) pg/mL. LV mass index and LV ejection fraction were 151.67±42.5 g/m2 and 57.48±12.9%, respectively. The mean extracellular water was 35.97±1.04%. Serum NT-proBNP levels correlated positively with LV mass index (r=0.768, p=0.01) and extracellular water (r=0.866, p=0.01) and negatively with LV ejection fraction (r=-0.808, p=0.01). Serum NT-proBNP levels significantly correlated with LV mass index, LV ejection fraction, and extracellular water. Therefore, serum NT-proBNP levels can be a clinical predictive marker for LV hypertrophy, LV dysfunction, and volume status in CAPD patients.
Cardiovascular diseases are the most life-threatening complications in patients with end-stage renal failure and their incidence is 30 times as common as that of subjects with normal renal functions1, 2). Left ventricular hypertrophy occurs in 75% of patients with end-stage renal failure, congestive heart failure in 40% and coronary artery disease in greater than 40%. Thus, the Kidney Disease Outcome Quality Initiatives (K/DOQI) guidelines recommend that regular ultrasonography should be performed in asymptomatic patients with end-stage renal failure3). However, since ultrasonography is expensive and requires the experienced medical professional personnel, N-terminal pro-brain natriuretic peptide (NT-proBNP) which is inexpensive and easy has been extensively studied. NT-pro BNP is a neurohormone secreted to the ventricle in response to the volume or pressure of the ventricle. Based on the studies that indicate that NT-proBNP increases in plasma in the cases of decreased ventricular function4, 5), NT-proBNP has been used as a clinical marker for diagnosis, severity, and prognosis. It has been reported that a close relationship exists between BNP concentrations and the diagnosis, prognosis, and severity of cardiac failure6). DeFilippi et al.7) reported a significant relationship between BNP concentrations and left ventricular hypertrophy and ischemic heart disease in patients with chronic renal failure who did not receive hemodialysis. The serum level of NT-proBNP increases according to the amount of decrease in renal functions in patients with chronic renal failure, but it represents functional disturbance and hypertrophy of the left ventricle (LV)8). Zoccali et al.9) suggested that the serum BNP level can be used as a valuable marker for a screening test of cardiovascular disease in patients on hemodialysis and without heart disease.
Clinical implications of the serum NT-proBNP level in patients on peritoneal dialysis have not yet been elucidated. This study was conducted to measure the serum NT-proBNP level, amount of extracellular fluid, LV ejection fraction (LVEF) and LV mass index (LVMI) in order to investigate their relationship and to determine whether NT-proBNP can be used as a predictive marker of heart disease such as left ventricular dysfunction in patients with renal failure on continuous peritoneal dialysis on an outpatient basis.
This single-center cross-sectional study included patients with end-stage renal failure who received continuous ambulatory peritoneal dialysis (CAPD) for greater than 1 year on an outpatient basis between February and October of 2006. The research data were obtained from patients who hadn't had peritonitis within the last 30 days and were steadily receiving 8 L of peritoneal dialysis a day (2 L, 4 times). Patients with acute ischemic heart disease, atrial fibrillation, or moderate or higher degree of valvular heart disease were excluded from this study. A total of 30 patients were enrolled in this study. There were 19 males (63.3%) and 11 females (36.7%), and the mean age was 47±12 years. The underlying diseases of renal failure were diabetes mellitus (n=14, 46.7%), hypertension (n=7, 23.3%) and glomerulonephritis (n=9, 30%).
All dialysate and urine were collected in the 24 hour time period, from the morning before the test to the actual morning. After all peritoneal dialysate were drained, blood tests, and bioimpedance analysis were done.
The serum NT-proBNP was measured by electrochem iluminescence immunoassay (Rocher Diagnostics, Indianapolis, IN, USA) with blood samples collected from fasting patients who were stabilized in a supine position for 30 minutes. LVEF, ventricular wall motion abnormality, interventricular septal thickness, ventricular posterior wall thickness, and LV diameter at end-diastole were measured using an M-mode, 2-dimensional cardiac sonograph (HDI 5000, Phillips, Bothell, WA, USA) and LV mass was calculated from the equation proposed by Devereux10):
LV mass (g)=1.04×[(IVSd+LVPWd+LVID)3-LVIDd3]-13.6.
Where IVSd (cm) is the thickness of interventricular septum, LVIDd (cm) is LV internal diameter, and LVPWd (cm) is the thickness of LV posterior wall.
LVMI was obtained by dividing LV mass by the body surface area. Resistance and impedance were measured by a total of 8 electrodes which were attached to both thumbs, both palms, both anterior portions of the soles of the feet and both posterior portions of the soles of the feet using a multifrequency bioimpedance analyzer (X-scan plus II, Jawon Medical, Gyeongsan, Korea).
Resistance and impedance measured between 1 KHz and 1 MKHz were applied in the following equation:
arm (A) RIA = arm length (cm)2/RA(O)
leg (L) RIL = leg length (cm)2/RL(O)
trunk (T) RIT = trunk height (cm)2/RT(O)
Whole body (sum)
RIsum=total body height(cm)2/RRA(O)+RLA(O)+RT(O)+RRL(O)+RLL(O)
These measurements were converted into percentages relative to total body water, intracellular water %, and extracellular water % (ECW%) by the Lukaski formula11) using bioimpedance analysis software.
All descriptive data were expressed as mean±SD and percentage. Statistical analyses were performed using SPSS for Windows 12.0. A p value of <0.05 was considered statistically significant. Although the serum level of NT-proBNP did not show a normal distribution, the level of logarithmically transformed NT-proBNP was found to have a normal distribution which was expressed as a median. The correlations between the serum NT-proBNP level and the aforementioned variables were determined using the Spearman's correlation analysis.
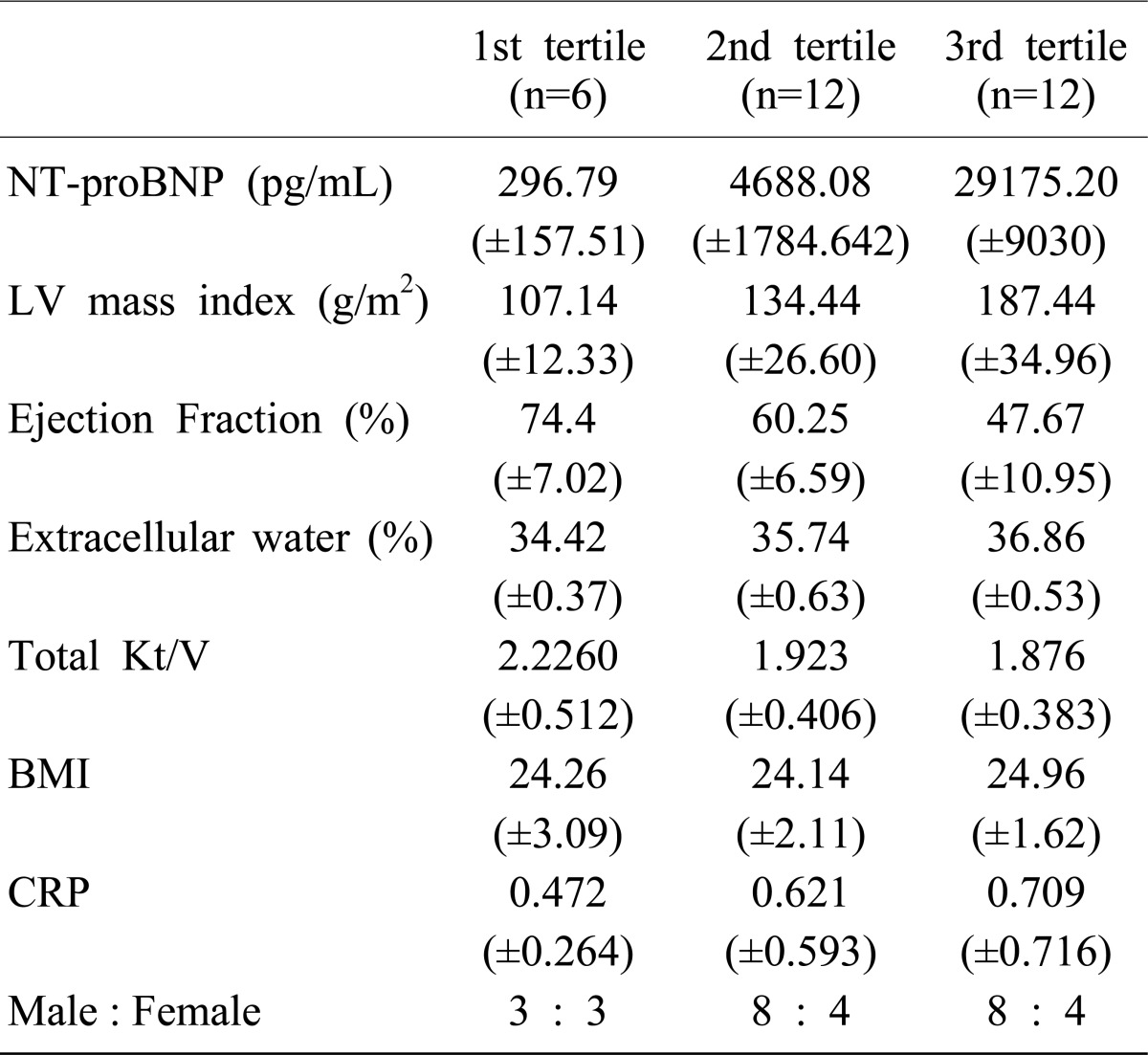
In a total of 30 patients with renal failure, the average age was 47 (range, 35-59) years old, and the proportion of gender was 19 male and 11 female. The cause of renal failure was diabetes mellitus in 14 patients (46.7%), hypertension in 7 patients (23.3%), and glomerular disease in 9 patients (30%). There were no patients taking special medicine and diuretics were not given to any of the patients. Average duration of CAPD was 57 years (17-84) and the occurrence rate for peritonitis was 2.3 times (0-4). The body mass index (BMI) was on average 24.5 kg/m2 (range, 19.6-28.5 kg/m2, ECW% 35.97% (range, 34.93-37.6%), LVMI 151.67 g/m2 (range, 85.3-273.0 g/m2, and LVEF 57.48% (range, 33-83%). Laboratory tests revealed hemoglobin 10.3 g/dL (range, 8.4-13.7 g/dL); albumin 3.2 g/dL (range, 2.9-3.9 g/dL); calcium 7.73 mg/dL (6.38-10.41 mg/dL); phosphate, 4.76 mg/dL (range, 2.4-7.5 mg/dL); C-reactive protein (CRP), 0.82 mg/dL (range, 0.32-2.38 mg/dL); blood urea nitrogen (BUN), 67.4 mg/dL (range, 35.4-105.1 mg/dL); creatinine, 6.6 mg/dL (range, 3.4-11.3 mg/dL); NT-proBNP, 14,236.56 pg/mL (range, 83-35,000 pg/mL), and weekly total Kt/Vurea 1.956 (range, 1.536-2.376) (Table 1).
The total volume, urea, and the creatinine level of the peritoneal dialysis fluid and urine that were drained within the 24 hour period were examined. Weekly total Kt/V was calculated according to the following method.
Weekly total Kt/V= daily Kt/V×7
Daily total Kt/V= (Kpt+Krt)/V
(Kpt: peritoneal clearance, Krt: residual renal clearance, V: total volume)
Patients were divided into 3 groups: group 1 with a range of 1-1000 pg/mL (n=6), group 2 with a range of 1,001-10,000 pg/mL (n=12), and group 3 with a range of 10,001-35,000 pg/mL (n=12). In group 1, the median was 296.8 pg/mL, the minimum was 83.15 pg/mL and the maximum was 516.3 pg/mL. In group 2, the median was 4,688.1 pg/mL, the minimum was 1,585 pg/mL and the maximum was 6,579 pg/mL. In group 3, the median was 29,175.3 pg/mL, the minimum was 12,621 pg/mL and the maximum was 35,000 pg/mL. In this study, patients were not classified according to age, sex, presence of diabetes mellitus or duration of peritoneal dialysis. Group 3 showed the highest LVMI, the highest ECW%, and the lowest LVEF. However, there were no significant differences in total Kt/V, BMI, CRP, or sex among the 3 groups. The three groups were examined using the Kruskal-Wallis test (Table 2).
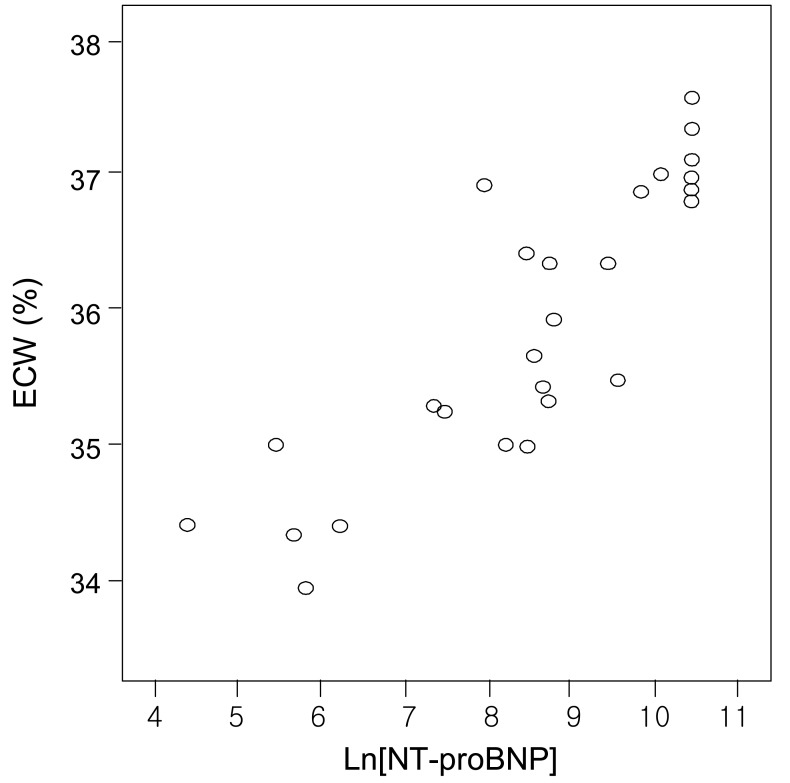
There was a positive correlation between the serum NT-proBNP level and either LVMI or ECW% (r=0.854, p=0.01; and r=0.857, p=0.01, respectively) (Fig. 1 and 3). The serum NT-proBNP level was negatively correlated with LVEF (r= -0.835, p=0.01) (Fig. 2). In this study, according to the criteria proposed by Framingham12), left ventricular hypertroply was defined as LVMI ≥131 g/m2 in males and ≥100 g/m2 in females, and normal LVEF was defined as 56%. Eighty-three percent of the 30 patients showed left ventricular hypertrophy and 70% functional disturbance of the left ventricle.
The serum NT-proBNP level tended to increase as LVMI and ECW% increased in patients with renal failure who underwent continuous peritoneal dialysis on an outpatient basis. Conversely, the serum NT-proBNP level tended to decrease as LVEF increased (Table 2).
The main causes of death in patients with end-stage renal failure are cardiovascular diseases which are more prevalent in such patients than in normal subjects primarily due to excessive body water and hypertension. Stoddard et al.13) have reported that left ventricular hypertrophy is the most common finding and a clinical marker predictive of poor prognosis. The causes of a decrease in left ventricular contraction and cardiac failure include hypertension, arteriosclerosis, pressure load due to aortic stenosis, arteriovenous aneurysm, sodium retention and excessive body water load, among which excessive body water load is the main cause14). Thus, in this study, in order to determine the prognosis of patients with end-stage renal failure, left ventricular hypertrophy, left ventricular failure, and excessive body water load were evaluated, and the serum NT-proBNP level was measured to determine whether the serum NT-proBNP can be used as a prognostic factor predictive of left ventricular hypertrophy and left ventricular failure.
ProBNP consists of 108 amino acids and is secreted by cardiac muscle cells of the atrium and ventricle but mainly by the left ventricle5). When it enters blood vessels, it dissolves into inactive NT-proBNP, which consists of 76 amino acids and active BNP containing 32 amino acids. Since NT-proBNP has a longer half life and is more stable in plasma or serum than BNP, it is more useful in clinical settings. Bay et al.5) have reported that the serum NT-proBNP level is a single factor predictive of left ventricular failure at initial evaluation. In addition, many studies have indicated that the serum NT-proBNP level can be a predictor of cardiac failure in normal subjects4, 7). Based on such studies, the serum NT-proBNP has been used as a valuable marker for the diagnosis, prognosis, and evaluation of treatment outcome of left ventricular failure.
Choi et al.8) have demonstrated that although the serum NT-proBNP level increases as renal functions decreases, it shows a significant correlation with left ventricular hypertrophy and systolic/diastolic disorders and that analysis using the receiver operating characteristic curve reveals an AUC value of 0.76-0.84 which has the potential to predict and screen left ventricular hypertrophy and functional disturbance. DeFilippi et al.7) have documented that an increase in the serum NT-proBNP level can be a predictive factor of cardiac disease in asymptomatic patients with chronic renal disease.
A previous study on the correlation between the serum NT-proBNP level and cardiac functions in patients on hemodialysis have suggested that the serum NT-proBNP level can be used as a marker for screening cardiovascular disease in patients without symptoms of cardiovascular disease9). Although many studies have reported that the serum NT-proBNP level has clinical implications in predicting cardiac disease in patients with chronic renal failure or those on hemodialysis, there have been few studies on the clinical implications of the serum NT-proBNP level in those on peritoneal dialysis. Thus, this study was conducted to determine the usefulness of the serum NTproBNP level in predicting cardiac disease in patients on peritoneal dialysis. As shown in this study, patients on peritoneal dialysis showed a higher NT-proBNP level than normal subjects, which may be attributed to a delay in hormone secretion due to a decrease in renal function, an increase in the volume of the left ventricle due to a long-term excess state of body water, left ventricular hypertrophy due to pressure load, and a high prevalence of ischemic heart disease and congestive heart failure8, 14). Since patients on peritoneal dialysis are in a persistent state of excess body water with resultant edema, left ventricular hypertrophy and congestive heat failure, the potential for left ventricular hypertrophy in such patients can be predicted by measuring ECW% which represents the state of excess body water. There have been many studies on the correlation between the serum NT-proBNP and body water load. Naganuma et al.15) have demonstrated that in patients on hemodialysis, the serum NT-proBNP level is related to changes in body weight and that since the serum NT-proBNP level is lower after hemodialysis than before hemodialysis, the serum NT-proBNP is related to the state of body water. Nishikimi et al.16) have reported that when they measured plasma BNP levels on Monday, Wednesday, and Friday before and after hemodialysis in 28 consecutive patients who underwent hemodialysis three times weekly, the plasma BNP level did not change after hemodialysis on Monday, but the plasma BNP level significantly decreased after hemodialysis on Wednesday and Friday, suggesting that the plasma BNP levels before and after hemodialysis in patients with chronic renal failure directly correlate with the degree of body fluid retention. Fagugli et al.17) reported in a bioimpedance study for the measurement of ECW% that although patients were on hemodialysis, there was no significant difference in the serum NT-proBNP level before and after hemodialysis and that there was a positive correlation between the serum NT-proBNP level and the degree of retention of body water. The reason for this may be that the heart increases in volume and stretches due to body water overload, which induces secretion of NT-proBNP from cardiac muscle cells of the atrium and ventricle18). In addition, it has been reported that since the serum NT-proBNP level represents the heart state before and after body water load in patients on hemodialysis, it can be used as a guideline for the prevention of cardiac disease in such patients19).
Lee et al.20) have demonstrated that LVMI significantly increases and LVEF significantly decreases as the serum NT-proBNP level increases in patients on peritoneal dialysis, but that ECW% does not change. In this study, as the serum NT-proBNP level increased in patients on continuous peritoneal dialysis on an outpatient basis, LVMI significantly increased and LVEF significantly decreased, suggesting that the serum NT-proBNP level represents left ventricular hypertrophy and functional disturbance, which was consistent with the results of Lee et al.20) In contrast to the results of Lee et al.20), as the serum NT-proBNP level increased, ECW% significantly increased, suggesting that the NT-proBNP level represents the excess state of body water in patients on peritoneal dialysis. When long-term body water overload persists in patients on continuous peritoneal dialysis, left ventricular hypertrophy develops, left ventricular functions decrease, and cardiac muscle cells stretch with a resultant increase in secretion of NT-proBNP. This increase in the serum NT-proBNP level may lead to an increase in LVMI and a decrease in LVEF.
In conclusion, the results of this study suggest that the serum NT-proBNP level can be used as a valuable marker predictive of left ventricular hypertrophy, ventricular contraction, and ECW% in patients on continuous peritoneal dialysis on an outpatient basis. Our results implicate the serum NT-proBNP level as a non-invasive method for screening cardiac disease and predicting their prognosis in patients with end-stage renal failure who are on peritoneal dialysis. Further studies with a larger sample size and with normal controls are needed to confirm our results.
References
1. Culleton BF, Larson MG, Wilson PW, Evans JC, Parfrey PS, Levy D. Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int. 1999; 56:2214–2219. PMID: 10594797.

2. Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998; 9:S16–S23. PMID: 11443763.
3. K/DOQI Workgroup. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patiets. Am J Kidney Dis. 2005; 45(Suppl 3):S1–S153.
4. Murdoch DR, Byrne J, Morton JJ, McDonagh TA, Robb SD, Clements S, et al. Brain natriuretic peptide is stable in whole blood and can be measured using a simple rapid assay: implications for clinical practice. Heart. 1997; 78:594–597. PMID: 9470878.

5. Bay M, Kirk V, Parner J, Hassager C, Nielsen H, Krogsgaard K, et al. NT-proBNP: a new diagnostic screening tool to differentiate between patients with normal and reduced left ventricular systolic function. Heart. 2003; 89:150–154. PMID: 12527664.

6. Kwon SH, On YK, Han DH, Lee SC, Jo YH, Lee NH, et al. Usefulness of B-type Natriuretic Peptide in Congestive Heart Failure. Korean Circ J. 2003; 33:695–700.

7. DeFilippi CR, Fink JC, Nass CM, Chen H, Christenson R. N-terminal pro-B-type natriuretic peptide for predicting coronary disease and left ventricular hypertrophy in asymptomatic CKD not requiring dialysis. Am J Kidney Dis. 2005; 46:35–44. PMID: 15983955.

8. Choi SY, Kim JA, Lee JE, Do YS, Jang EH, Bae HJ, et al. Plasma Levels of N-terminal pro-brain Natriuretic Peptide (NT-proBNP) and Left Ventricular Function in Patients with Chronic Renal Failure. Korean J Nephrol. 2006; 25:413–421.
9. Zoccali C, Mallamaci F, Benedetto FA, Tripepi G, Parlongo S, Cataliotti A, et al. Cardiac natriuretic peptides are related to left ventricular mass and function and predict mortality in dialysis patients. J Am Soc Nephrol. 2001; 12:1508–1515. PMID: 11423580.

10. Ganau A, Devereux RB, Roman MJ, de Simone G, Pickering TG, Saba PS, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992; 19:1550–1558. PMID: 1534335.

11. Snel YE, Brummer RJ, Doerga ME, Zelissen PM, Koppeschaar HP. Validation of extracellular water determination by bioelectrical impedance analysis in growth hormone-deficient adults. Ann Nutr Metab. 1995; 39:242–250. PMID: 8546441.

12. Levy D, Savage DD, Garrison RJ, Anderson KM, Kannel WB, Castelli WP. Echocardiographic criteria for left ventricular hypertrophy: the Framingham Heart Study. Am J Cardiol. 1987; 59:956–960. PMID: 2952002.

13. Stoddard MF, Pearson AC, Kern MJ, Ratcliff J, Mrosek DG, Labovitz AJ. Influence of alteration in preload on the pattern of left ventricular diastolic filling as assessed by Doppler echocardiography in humans. Circulation. 1989; 79:1226–1236. PMID: 2498005.

14. Brun P, Tribouilloy C, Duval AM, Iserin L, Meguira A, Pelle G, et al. Left ventricular flow propagation during early filling is related to wall relaxation: a color M-mode Doppler analysis. J Am Coll Cardiol. 1992; 20:420–432. PMID: 1634681.

15. Naganuma T, Sugimura K, Wada S, Yasumoto R, Sugimura T, Masuda C, et al. The prognostic role of brain natriuretic peptides in hemodialysis patients. Am J Nephrol. 2002; 22:437–444. PMID: 12381941.

16. Nishikimi T, Futoo Y, Tamano K, Takahashi M, Suzuki T, Minami J, et al. Plasma brain natriuretic peptide levels in chronic hemodialysis patients: influence of coronary artery disease. Am J Kidney Dis. 2001; 37:1201–1208. PMID: 11382689.

17. Fagugli RM, Palumbo B, Ricciardi D, Pasini P, Santirosi P, Vecchi L, et al. Association between brain natriuretic peptide and extracellular water in hemodialysis patients. Nephron Clin Pract. 2003; 95:C60–C66. PMID: 14610331.

18. Su X, Brower G, Janicki JS, Chen YF, Oparil S, Dell'Italia LJ. Differential expression of natriuretic peptides and their receptors in volume overload cardiac hypertrophy in the rat. J Mol Cell Cardiol. 1999; 31:1927–1936. PMID: 10525429.

19. Dastoor H, Bernieh B, Boobes Y, Abouchacra S, Eltayeb E, Elhuda MN, et al. Plasma BNP in patients on maintenance haemodialysis: a guide to management? J Hypertens. 2005; 23:23–28. PMID: 15643118.
20. Lee JA, Kim DH, Yoo SJ, Oh DJ, Yu SH, Kang ET. Association between serum n-terminal pro-brain natriuretic peptide concentration and left ventricular dysfunction and extracellular water in continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 2006; 26:360–365. PMID: 16722030.

Fig. 1
Correlation between logarithmically transformed values of N-terminal pro-brain natriuretic peptide (Ln[NT-proBNP]) and left ventricular mass index (LVMI) (r=0.854, p=0.01).
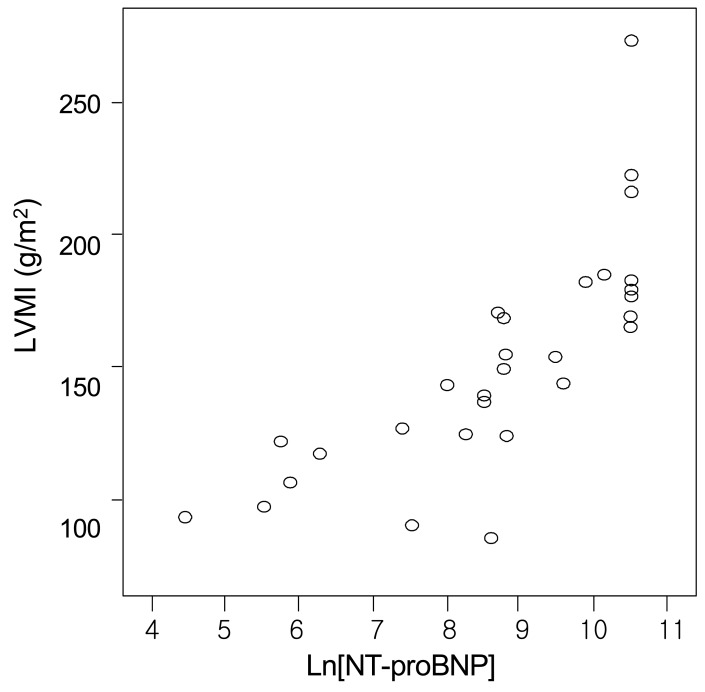
Fig. 2
Correlation between logarithmically transformed values of N-terminal pro-brain natriuretic peptide (Ln[NT-pro BNP]) and ejection fraction (EF) (r=-0.835, p=0.01).
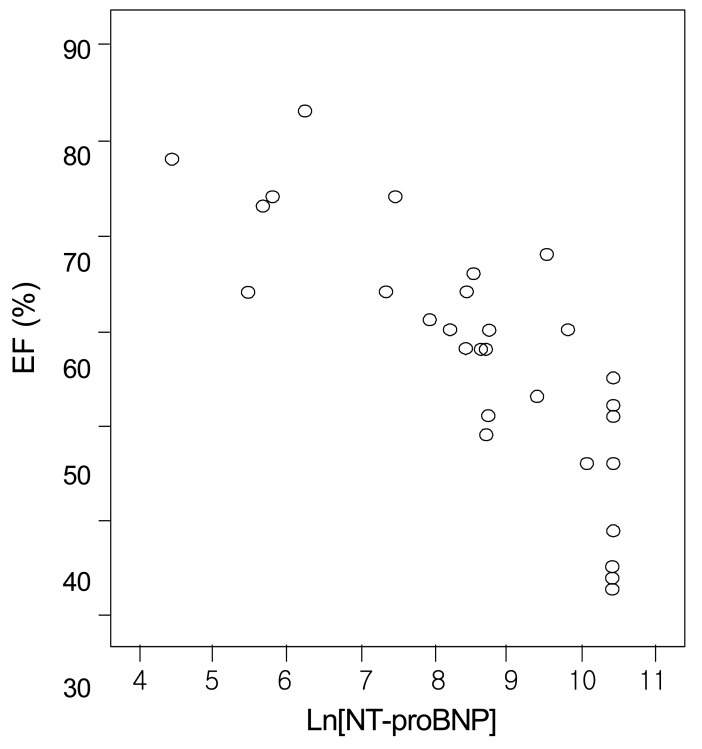
Fig. 3
Correlation between logarithmically transformed values of N-terminal pro-brain natriuretic peptide (Ln[NT-pro BNP]) and extracellular water (ECW) (r=0.857, p=0.01).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download