Abstract
The deoxycorticosterone acetate (DOCA)-salt rat is known as a model of volume dependent hypertension and characterized by increased cardiac endothelin-1 (ET-1) content. Recently, it has been reported that rosiglitazone (RGT), a peroxisome proliferator-activated subtype gamma receptor agonist, shows blood pressure lowering effect. We investigated whether DOCA-salt hypertension is associated with altered expression of heat shock proteins (HSP) and ET-1 in the heart, aorta, and kidney, and whether RGT changes HSP expression and ET-1 in association with its blood pressure lowering effect. Two weeks after the silastic DOCA (200 mg/kg) strips implantation, DOCA-salt rats were randomly divided to receive control diet with or without RGT (10 mg/kg/day) for another 2 weeks. The mRNA expression of ET-1 was determined by real time polymerase chain reaction. The expression of HSP was determined by semiquantitative immunoblotting. In DOCA-salt rats, systolic blood pressure was markedly increased, while creatinine clearance decreased. RGT treatment attenuated high blood pressure and decreased creatinine clearance in DOCA-salt rats. The mRNA expression of ET-1 was increased in DOCA-salt rats compared to controls, which was counteracted by RGT treatment. The protein expression of HSP70, HSP32, and HSP25 was increased in the kidney and heart in DOCA-salt rats, which was attenuated by RGT treatment in the kidney, but not in the heart. In conclusion, increased expression of ET-1 may play a role in the pathogenesis of hypertension in DOCA-salt rats, which was counteracted by the treatment of RGT. Up-regulation of HSP70, HSP32, and HSP25 in the kidney and heart may play a role in organ protection against a variety of stresses.
Most investigators who are interested in the pathogenesis of hypertension agree that it is not a homogenous process, but rather heterogenous involving multiple and different processes. For that reason, Laragh and his cowokors suggested the concept of "volume and vasoconstriction" in the pathogenesis of hypertension1). The Deoxycorticosterone acetate (DOCA) treated animals had elevated plasma sodium and osmolality levels2), which is consistent with the observations that this model of hypertension is associated with increased renal sodium and water reabsorption3) and with the expansion of extracellular fluid volume4). The mechanism by which the abnormality in sodium homeostasis raises blood pressure remains unclear, but must involve an element of vasoconstriction.
There is accumulating evidence indicating that endothelin-1 (ET-1) plays an important role in the development of hypertension. ET-1 is a potent vasoconstrictor, as well as a mediator of smooth muscle mitogenesis, and thus, a determinant of systolic blood pressure5). ET-1 content and its mRNA expression are elevated in vascular and renal tissues of DOCA-salt hypertensive rats6). These findings suggest that ET-1 contributes to the development of high blood pressure and vascular growth in DOCA-salt hypertensive rats7).
Heat shock proteins (HSP) may be induced in response to a variety of stresses, including hypertension. The cellular expression of major HSP gene is increased in response to heat exposure in hypertensive animals and humans8), and has been known to possess a protective role in various pathphysiologic states. The expression of HSP32/heme oxygenase (HO)-1 is increased in several tissues in response to angiotensin II infusion, along with increased blood pressure9, 10). An induction of HSP70 plays a protective role in the vasculature from hemodynamic stress11), and HSP25 protect cells from heat shock and oxidative stress when overexpressed12). Furthermore, altered regulation of HSP has been implicated in the development and maintenance of hypertension. A prolonged HSP32 induction counteracts the increased blood pressure in renovascular hypertension13).
Thiazolidinediones (TZDs) are used to treat type 2 diabetes because of their efficacy in controlling blood glucose secondary to enhancing insulin action through a mechanism that is yet to be completely elucidated14). These compounds are also capable of a rich array of beneficial effects, including reductions of in blood pressure in animal models and humans15-17). TZD treatment has been shown to attenuate the release of endothelin-1 from bovine vascular cells, which may contribute to the observed blood pressure lowering effect18). Thus, it may be possible that antihypertensive effect of rosiglitazone is caused by changes of endothelin system in DOCA-salt hypertensive rats. In addition, rosiglitazone treatment inhibits apoptosis and decreases protein expression of HSP60 in vascular endothelial cells, which may have beneficial vascular effects19). In this context, the blood pressure lowering effects of TZD may be associated with the changes of HSP and endothelin system in the vasculature and kidney.
The present study was aimed to investigate whether antihypertensive effects of rosiglitazone in DOCA-salt hypertensive rats is associated with altered regulation of endothelin system and HSP isoforms in the kidney and heart in order to elucidate the underlying molecular mechanisms responsible for the pathogenesis of hypertension.
Male Sprague-Dawley rats weighing 180 to 200 g were used. The experimental procedure conformed to the institutional guidelines for experimental animal care and use. Three groups of rats were prepared. One week after left unilateral nephrectomy, DOCA-salt rats were subcutaneously implanted with silastic DOCA (200 mg/kg) strips. The control group was also left unilaterally nephrectomized but were without DOCA implantation. Physiologic saline was supplied as a drinking water to all animals. Two weeks after the operation, DOCA-salt rats were randomly divided to receive control diet with or without rosiglitazone (RGT, 10 mg/kg/day) for another 2 weeks. On the 2nd and 4th week after DOCA implantation, systolic blood pressure was measured by the tail cuff method (Pressure Meter, Model LE 5001; Panlab S. L., Barcelona, Spain). During the last 3 days, rats were maintained in the metabolic cages, allowing quantitative urine collections and measurements of water intake. Urine volume, creatinine, and sodium concentrations were measured. Plasma was collected from the inferior vena cava at the time of sacrifice, and analyzed for the measurement of creatinine, and sodium concentrations.
Another set of animal experiments had been done for the assay of real time polymerse chain reaction (PCR). On the experimental day, the rats were decapitated under a conscious state and trunk blood was taken. The kidney and aorta were rapidly taken and kept at -70℃ until assayed for the real time PCR.
The whole kidney and heart were homogenized at 25,000 rpm in a solution containing 250 mM sucrose, 1 mM ethylenediaminetetraacetate, 0.1 mM phenylmethylsulfonyl fluoride, and 10 mM Tris-HCl buffer (pH 7.6). Large tissue debris and nuclear fragments were removed by centrifugations (1,000×g for 20 min). Then the supernatant was pipetted off and kept on ice. Protein concentrations were measured by the bicinchoninic acid Assay Kit (Bio-Rad, Hercules, CA, USA).
All samples were adjusted with isolation solution to reach the same final protein concentrations, solubilized at 65℃ for 15 min in SDS-containing sample buffer and then stored at -20℃. To confirm equal loading of protein, an initial gel was stained with Coomassie blue. SDS-PAGE was performed on 6 or 12.5% polyacrylamide gels. The proteins were transferred by gel electrophoresis (Bio-Rad Mini Protean II Cell, Hercules, CA, USA) onto nitrocellulose membranes (Hybond ECL RPN3032D, Amersham Pharmacia Biotech, Little Chalfont, UK). The blots were subsequently blocked with 5% milk in PBS-T (80 mM Na2HPO4, 20 mM NaH2PO4, 100 mM NaCl, 0.1% Tween 20, pH 7.5) for 1 h. They were then incubated with affinity-purified anti-mouse monoclonal antibodies against HSP70 (1:1,000), anti-mouse polyclonal antibodies against HSP32 (1:1,000), or anti-rabbit polyclonal antibodies against HSP25 (1:1,000) in 0.2% nonfat milk/TBST for overnight at 4℃. The antibodies were purchased from stressGen (Victoria, BC, Canada). The membranes were again incubated with a horseradish peroxidase-labeled goat anti-rabbit IgG (1:2,000) in 2% nonfat milk/TBST for 1hr. The labeling was visualized by enhanced chemiluminescence (Amersham, Buckinghamshire, UK) and Image Reader (LAS-3000 Imaging System, Fuji Photo Film, Tokyo, Japan).
The kidney and aorta was homogenized in Trizol reagent (Invitrogen, Carlsbad, CA, USA). RNA was extracted with chloroform, precipitated with isopropanol, washed with 75% ethanol, and then dissolved in distilled water. The RNA concentration was determined by the absorbance read at 260 nm (Ultraspec 2000; Pharmacia Biotech, Cambridge, UK).
The expression of endothelin-1 (ET-1: 5'-CACAGGTAGACCCTAAGAGTCACAAG-3' and 5'-CTGTTGGACCACTGAATCCTGCCGATG-3'), type A endothelin receptor (ETAR : 5'-GACCACAATGATTTTGGAGTG-3' and 5'-GAACCAGCACCGCAACTCGTA-3') and type B endothelin receptor (ETBR: 5'-ACTGGCCATTTGGAGCTGAGA T-3' and 5'-GACGTATGGTGAAAAGAAAGAC-3') mRNA was determined by real-time PCR. cDNA was made by reverse transcribing 5 µg of total RNA using oligo (dT) priming and superscript reverse transcriptase II (Invitrogen, Carlsbad, CA, USA). cDNA was quantified using the Smart Cycler II System (Cepheid, Sunnyvale, CA, USA) and SYBR Green was used for detection. Each PCR reaction was done in 0.4 µM forward primer, 0.4 µM reverse primer, 2X SYBR Green Premix Ex Taq (TAKARA BIO INC, Seta 3-4-1, Otsu, Japan) 10 µM, 0.5 µL cDNA and HAO to bring the final volume to 20 µL. PCR was done using an the Rotor-GeneTM 3000 Detector System (Corbette research, Mortlake, New South Wales, Australia). Primers were prepared as described previously20). Following steps were taken : 1) 95℃ for 5 min; 2) 95℃ for 20 s; 3) 58 to 62℃ for 20 s (optimized for each primer pair); 4) 72℃ for 30 s; and 5) 85℃ for 6 s to detect SYBR Green. Steps 2-5 were repeated for additional 45 cycles, while at the end of the last cycle temperature was increased from 60 to 95℃ to produce a melt curve. Data from the reaction were collected and analyzed with the Corbett Research Software. The comparative critical threshold values from quadruplicate measurements were used to calculate the gene expression, with normalization to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control21). Melting curve analysis was performed to enhance specificity of the amplification reaction.
Drugs were purchased from Sigma Chemical Co. (St. Louis, MO, USA), unless stated otherwise. Results are expressed as mean±SEM. Multiple comparisons among the groups were determined by one-way ANOVA and post hoc Tukey HSD test. p values<0.05 were considered significant.
As shown in Fig. 1, systolic blood pressure (SBP) was markedly increased in DSH rats after 4 weeks of treatment, compared with control rats (241.1±13.1 vs 155.1±9.4 mmHg, p<0.05). RGT attenuated increased SBP (212.6±29.3 mmHg, p<0.05) in DOCA-salt rats. Kidney weights and left ventricular weights were increased in DOCA-salt rats as compared with controls, which were attenuated by RGT treatment. The creatinine clearance was decreased in DOCA-salt rats, which was attenuated by RGT treatment (Table 1).
Fig. 2 shows the expression of ET-1, ETAR and ETBR in the kidney and aorta. The abundance of ET-1 mRNA was significantly increased in the kidney and aorta of DOCA-salt rats compared with controls, which was counteracted by RGT treatment. The expression of ETAR was decreased in the kidney of DOCA-salt rats and increased in the aorta of DOCA-salt rats, which was not further affected by RGT treatment. The expression of ETBR was increased in the kidney and aorta of DOCA-salt rats, which was conteracted by RGT treatment.
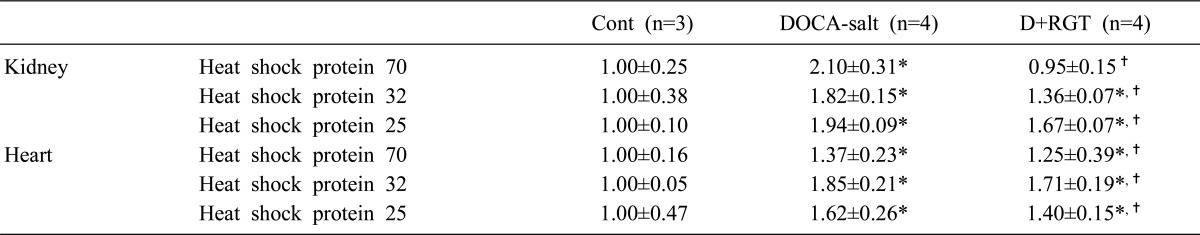
Fig. 3 and Table 2 shows the protein expression of HSP70, HSP32, and HSP25 in the kidney and heart. The expression of HSP70, HSP32, and HSP25 was increased in the kidney of DOCA-salt rats, which was counteracted by RGT treatment. The expression of HSP70, HSP32, and HSP25 was also increased in the heart, which was further affected by RGT treatment.
ET-1 not only induced vasoconstriction and has been demonstrated to play a role in renal and cardiac target organ damage22). The present study demonstrated that ET-1 mRNA expression was increased in the aorta and kidney associated with the increased blood pressure and decreased GFR. These findings may suggest that enhanced vascular and renal production of ET-1 play a role in the development of hypertension and renal dysfunction. These results are in line with the previous observations that chronic administration of a selective endothelin ETAR antagonist or nonselective endothelin ETAR/ETBR antagonist to DOCA-salt rats suppress the development of hypertension, vascular hypertrophy and renal injury23, 24). However, the mechanism by which ET-1 production is enhanced in blood vessels and kidneys in DOCA-salt rats are unknown. Recent study demonstrated that incubation with xanthine/xanthine oxidase or H2O2 augments ET-1 mRNA levels in human mesangial cells25). These findings suggest that oxidant stress stimulates endothelin-1 production at a stage of its gene expression.
ET-1 produces its effects by acting on specific subtypes of receptors, ETAR and ETBR, which receptors are expressed mainly in the vascular tissues, in which it mediates the vasoconstrictor and growth effects26, 27). The ETBR also exist on endothelial cells, where they mediate vasodilation via the release of endothelium-derived nitric oxide28). In the present study, the mRNA expression of ETAR was decreased in kidney, while increased in the aorta of DOCA-salt rats. The down-regulation of ETAR may play a compensatory role in the vasoconstriction by ET-1 in kidney, and up-regulated ETAR in the vasculature may contribute to the development of hypertension. The differential expression of ETAR in aorta and kidney may suggest the tissue specific regulation of ET receptor system. The up-regulation of ETBR may play a compensatory role in the vasoconstriction by ET-1 in kidney and aorta via the release of endothelium-derived nitric oxide.
Sustained high blood pressure is a powerful determinant in the development of cardiac and renal hypertrophy29). The present study revealed that DOCA-salt hypertensive rats showed increased cardiac weight and kidney weight indices compared to normotensive control rats. In addition, demonstrated that rosiglitazone decreased blood pressure and attenuated left ventricular hypertrophy in DOCA-salt rats. Thus, we found that an association between high blood pressure, and cardiac and renal hypertrophy exist in this model of hypertension, which was ameliorated by rosiglitazone treatment. In addition, blood pressure lowering effects of rosiglitazone were accompanied by decreased ET-1 mRNA expression in aorta and kidney as well as increased creatinine clearance in DOCA-salt rats. Thus, rosiglitazone appears to suppress the enhanced endothelin-1 production in vascular and renal tissues and the consequent development of hypertension, vascular hypertrophy and renal injury in this model of hypertension.
Alterations of HSP has been suggested to play a protective role against potential tissue damage. Molecular adaptation of vascular endothelial cells to oxidative stress is accompanied by an induction of HSP. An induction of HSP70 has a protective effect on the vasculature from hemodynamic stress11). The inducible form of HSP70 is shown to be regulated in the kidney in response to stimuli such as ischemia30) and hyperthermia31). Overexpression of transfected HSP70 in the renal cells was found to have a protective action against cisplatin toxicity and oxidative injury32). Induction of renal HSP32 has been shown to ameliorate renal injury induced by rhabdomyolysis33). Gene ablation of HSP32 exacerated cisplatin-induced renal tubular injury34). Thus, renal HSP32 is thought to play a role in protecting renal function against renal insults. The small HSP from human HSP27 and mouse HSP25 form large oligomers which can act as molecular chaperones in vitro, and protect cells from heat shock and oxidative stress when overexpresssed12). In addition, angiotensin II infusion induces renal HSP70 and HSP25, as well as HSP329). These findings may suggest the beneficial and compensatory role of HSP70, HSP32 and HSP25. In the present study, expression of HSP isoforms was increased in heart and kidney of DOCA-salt rats, which may play a protective role in the pathogenesis of hypertension and renal damage. Interestingly, RGT treatment attenuates expression of HSP in kidney, but not in heart. This discrepancy of HSP expression may represent that HSP expresion can be regulated by at the level of post-translation by cell specific cofactors in the vasculature and kidney. This remains to be further elucidated.
In summary, RGT treatment decreased blood pressure and impaired renal function in DOCA-salt hypertensive rats. Up-regulation of mRNA expression of ET-1 in the aorta and kidney play a role in the pathogenesis of development of hypertension in DOCA-salt rats, which was counteracted by the treatment of RGT. Up-regulation of HSP70, HSP32 and HSP25 may play a role in organ protection against to a variety of stress.
Acknowledgements
This study was supported by the Korean Society of Electrolyte and Blood Pressure Research Grant (2007) and Chonnam National University Hospital Research Institute of Clinical Medicine (CRI 080-40-1).
References
1. Laragh JH. Vasoconstriction-volume analysis for understanding and treating hypertension: the use of renin and aldosterone profiles. Am J Med. 1973; 55:261–274. PMID: 4355699.

2. Trinder D, Phillips PA, Risvanis J, Stephenson JM, Johnston CI. Regulation of vasopressin receptors in deoxycorticosterone acetate-salt hypertension. Hypertension. 1992; 20:569–574. PMID: 1398892.

3. Jeffries WB, Wang Y, Pettinger WA. Enhanced vasopressin (V2-receptor)-induced sodium retention in mineralocorticoid hypertension. Am J Physiol. 1988; 254:F739–F746. PMID: 2834967.

4. Kunes J, Nedvidek J, Zicha J. Vasopressin and water distribution in rats with DOCA-salt hypertension. J Hypertens Suppl. 1989; 7(Suppl 6):S204–S205. PMID: 2632718.

5. Miller RC, Pelton JT, Huggins JP. Endothelins--from receptors to medicine. Trends Pharmacol Sci. 1993; 14:54–60. PMID: 8480375.

6. Lariviere R, Thibault G, Schiffrin EL. Increased endothelin-1 content in blood vessels of deoxycorticosterone acetate-salt hypertensive but not in spontaneously hypertensive rats. Hypertension. 1993; 21:294–300. PMID: 8478038.

7. Larouche I, Schiffrin EL. Cardiac microvasculature in DOCA-salt hypertensive rats : effect of endothelin ET(A) receptor antagonism. Hypertension. 1999; 34:795–801. PMID: 10523363.
8. Hamet P, Malo D, Tremblay J. Increased transcription of a major stress gene in spontaneously hypertensive mice. Hypertension. 1990; 15:904–908. PMID: 2351441.

9. Ishizaka N, Aizawa T, Ohno M, Usui Si S, Mori I, Tang SS, et al. Regulation and localization of HSP70 and HSP25 in the kidney of rats undergoing long-term administration of angiotensin II. Hypertension. 2002; 39:122–128. PMID: 11799090.

10. Aizawa T, Ishizaka N, Taguchi J, Nagai R, Mori I, Tang SS, et al. Heme oxygenase-1 is upregulated in the kidney of angiotensin II-induced hypertensive rats : possible role in renoprotection. Hypertension. 2000; 35:800–806. PMID: 10720598.
11. Xu Q, Li DG, Holbrook NJ, Udelsman R. Acute hypertension induces heat-shock protein 70 gene expression in rat aorta. Circulation. 1995; 92:1223–1229. PMID: 7648669.

12. Rogalla T, Ehrnsperger M, Preville X, Kotlyarov A, Lutsch G, Ducasse C, et al. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J Biol Chem. 1999; 274:18947–18956. PMID: 10383393.
13. Botros FT, Schwartzman ML, Stier CT Jr, Goodman AI, Abraham NG. Increase in heme oxygenase-1 levels ameliorates renovascular hypertension. Kidney Int. 2005; 68:2745–2755. PMID: 16316349.

14. Day C. Thiazolidinediones: a new class of antidiabetic drugs. Diabet Med. 1999; 16:179–192. PMID: 10227562.

15. Dubey RK, Zhang HY, Reddy SR, Boegehold MA, Kotchen TA. Pioglitazone attenuates hypertension and inhibits growth of renal arteriolar smooth muscle in rats. Am J Physiol. 1993; 265:R726–R732. PMID: 8238439.

16. Gerber P, Lubben G, Heusler S, Dodo A. Effects of pioglitazone on metabolic control and blood pressure: a randomised study in patients with type 2 diabetes mellitus. Curr Med Res Opin. 2003; 19:532–539. PMID: 14594526.

17. Raji A, Seely EW, Bekins SA, Williams GH, Simonson DC. Rosiglitazone improves insulin sensitivity and lowers blood pressure in hypertensive patients. Diabetes Care. 2003; 26:172–178. PMID: 12502676.

18. Satoh H, Tsukamoto K, Hashimoto Y, Hashimoto N, Togo M, Hara M, et al. Thiazolidinediones suppress endothelin-1 secretion from bovine vascular endothelial cells: a new possible role of PPARgamma on vascular endothelial function. Biochem Biophys Res Commun. 1999; 254:757–763. PMID: 9920814.
19. Artwohl M, Holzenbein T, Furnsinn C, Freudenthaler A, Huttary N, Waldhausl WK, et al. Thiazolidinediones inhibit apoptosis and heat shock protein 60 expression in human vascular endothelial cells. Thromb Haemost. 2005; 93:810–815. PMID: 15886792.

20. Bae EH, Kim IJ, Park JW, Ma SK, Choi KC, Lee JU, et al. Altered regulation of renin-angiotensin, endothelin and natriuretic peptide systems in rat kidney with chronic unilateral ureteral obstruction. Urol Int. 2007; 79:170–176. PMID: 17851289.

21. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408. PMID: 11846609.
22. Schiffrin EL. Vascular endothelin in hypertension. Vascul Pharmacol. 2005; 43:19–29. PMID: 15955745.

23. Li JS, Turgeon A, Schiffrin EL. Effect of chronic treatment with two different ET(A) selective endothelin receptor antagonists on blood pressure and small artery structure of deoxycorticosterone acetate (DOCA)-salt hypertensive rats. Am J Hypertens. 1998; 11:554–562. PMID: 9633791.

24. Schiffrin EL, Sventek P, Li JS, Turgeon A, Reudelhuber T. Antihypertensive effect of an endothelin receptor antagonist in DOCA-salt spontaneously hypertensive rats. Br J Pharmacol. 1995; 115:1377–1381. PMID: 8564194.

25. Hughes AK, Stricklett PK, Padilla E, Kohan DE. Effect of reactive oxygen species on endothelin-1 production by human mesangial cells. Kidney Int. 1996; 49:181–189. PMID: 8770966.

26. Kohan DE, Fiedorek FT Jr. Endothelin synthesis by rat inner medullary collecting duct cells. J Am Soc Nephrol. 1991; 2:150–155. PMID: 1954327.

27. Terada Y, Tomita K, Nonoguchi H, Marumo F. Different localization of two types of endothelin receptor mRNA in microdissected rat nephron segments using reverse transcription and polymerase chain reaction assay. J Clin Invest. 1992; 90:107–112. PMID: 1321837.

28. Hirata Y, Emori T, Eguchi S, Kanno K, Imai T, Ohta K, et al. Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. J Clin Invest. 1993; 91:1367–1373. PMID: 7682570.

29. Frohlich ED, Apstein C, Chobanian AV, Devereux RB, Dustan HP, Dzau V, et al. The heart in hypertension. N Engl J Med. 1992; 327:998–1008. PMID: 1518549.

30. Smoyer WE, Ransom R, Harris RC, Welsh MJ, Lutsch G, Benndorf R. Ischemic acute renal failure induces differential expression of small heat shock proteins. J Am Soc Nephrol. 2000; 11:211–221. PMID: 10665928.

31. Manzerra P, Rush SJ, Brown IR. Tissue-specific differences in heat shock protein hsc70 and hsp70 in the control and hyperthermic rabbit. J Cell Physiol. 1997; 170:130–137. PMID: 9009141.

32. Komatsuda A, Wakui H, Oyama Y, Imai H, Miura AB, Itoh H, et al. Overexpression of the human 72 kDa heat shock protein in renal tubular cells confers resistance against oxidative injury and cisplatin toxicity. Nephrol Dial Transplant. 1999; 14:1385–1390. PMID: 10382997.

33. Nath KA, Balla G, Vercellotti GM, Balla J, Jacob HS, Levitt MD, et al. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992; 90:267–270. PMID: 1634613.

34. Shiraishi F, Curtis LM, Truong L, Poss K, Visner GA, Madsen K, et al. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am J Physiol Renal Physiol. 2000; 278:F726–F736. PMID: 10807584.
Fig. 1
Time course measurements of systolic blood pressure (SBP) determined by the tail-cuff method at 2 and 4 weeks of treatment. Symbols are: (◆) control; (■) deoxycorticosterone acetate-salt; (▲) rosiglitazone treated rats. Each point represents mean±SEM of experimental rats.
*p<0.05 compared with control.
#p<0.05 compared with DOCA-salt group.

Fig. 2
A) Expression of endothelin-1 (ET-1), type A endothelin receptor (ETAR) and type B endothelin receptor (ETBR) mRNA in the whole kidney. B) Expression of ET-1, ETAR and ETBR mRNA in the aorta. Columns show densitometric data representing control, deoxycorticosterone acetate (DOCA)-salt and rosiglitazone treatment (D+RGT) group.
*p<0.05 compared with control (Cont).
#p<0.05 compared with DOCA-salt group.
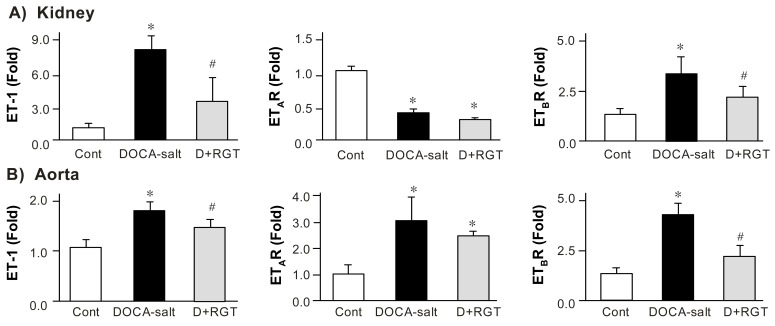
Fig. 3
Semiquantitative immunoblotting of heat shock protein 70 (HSP70) in the kidney and heart. The immunoblot was reacted with anti-HSP70. Densitometric analysis revealed increased expression of HSPs in deoxycorticosterone acetate (DOCA)-salt rats. Rosiglitazone treatment (D+RGT) prevented overexpression of HSP70 in the kidney of DOCA-salt rats.
*p<0.05 compared with control (Cont).
#p<0.05 compared with DOCA-salt group.
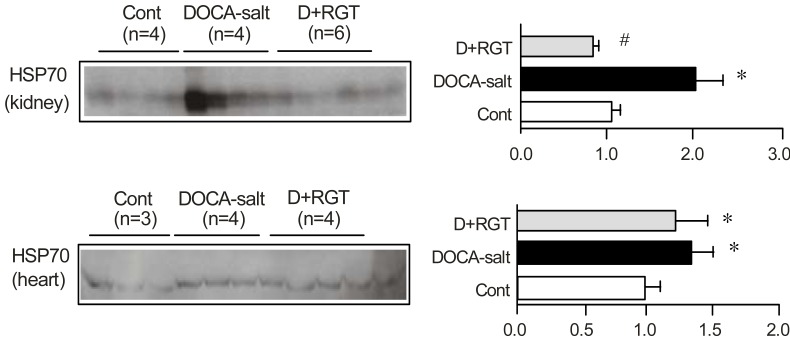
Table 1
Changes in Functional Data
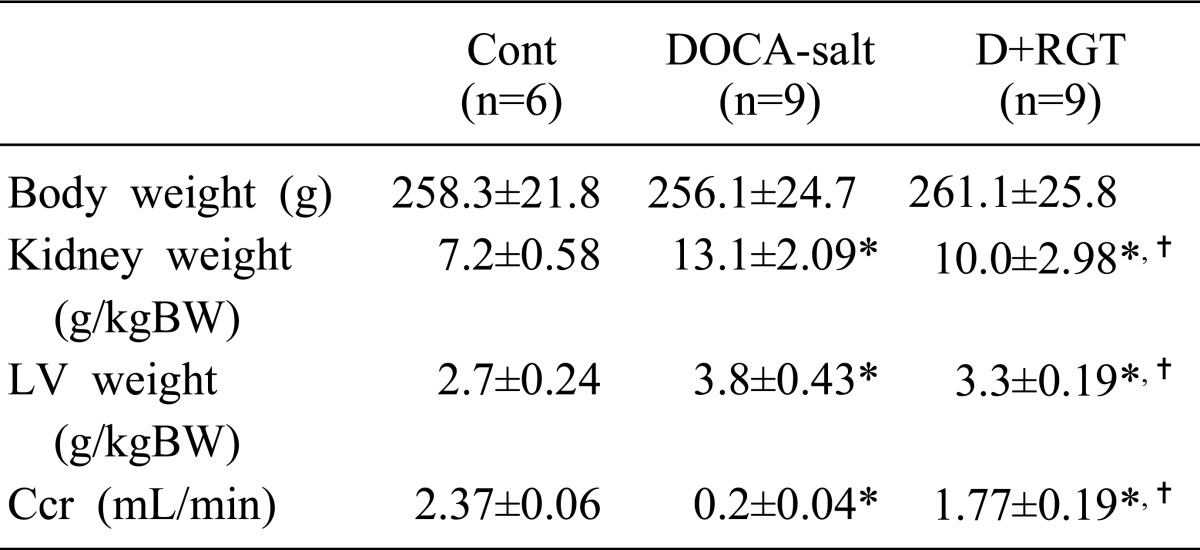
Values are expressed as mean±SE. These values are measured at the last day of experiments (week 4). DOCA, deoxycorticosterone acetate; D+RGT, rosiglitazone treatment in DOCA-salt rats; BW, body weight; LV, left ventricle; Ccr, creatinine clearance.
*p<0.05 when DOCA-salt or D+RGT groups were compared with control group (cont).
†p<0.05 compared with DOCA-salt group.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download