Abstract
The present study was aimed at investigating the role of type II 11β-hydroxysteroid dehydrogenase (IIβ-HSD II) in the development of hypertension. Two-kidney, one-clip (2K1C), deoxycorticosterone acetate (DOCA)/salt, or NG-nitro-L-arginine methyl ester (L-NAME) hypertension was induced in male Sprague-Dawley rats. Four weeks later, the expression of 11β-HSD II mRNA was determined in the kidney by Northern blot analysis. The plasma level of aldosterone was measured by radioimmunoassay. In 2K1C hypertension, the expression of 11β-HSD II was decreased in the clipped kidney and increased in the non-clipped kidney. The expression was increased in the remnant kidney of DOCA/salt hypertension, while decreased in the kidneys of L-NAME hypertension. The plasma level of aldosterone was increased, decreased, and remained unchanged in 2K1C, DOCA/salt, and L-NAME hypertension, respectively. The down-regulation of 11β-HSD II may contribute to the sodium retention, thereby increasing the blood pressure in 2K1C and L-NAME hypertension. On the contrary, the up-regulation in DOCA/salt hypertension may play a compensatory role to dissipate the sodium retention.
Mineralocorticoids activate epithelial sodium channels in the distal nephron of the kidney through binding to mineralocorticoid receptors (MR), thereby promoting sodium reabsorption1, 2). Since glucocorticoids may also bind to MR and exert a mineralocorticoid-like action, some protection of MR from their promiscuous access may be needed. The selective binding of mineralocorticoids to MR depends on an enzyme, 11β-hydroxysteroid dehydrogenase (11β-HSD), which catalyzes the conversion of cortisol in humans and corticosterone in rodents to their derivatives with low affinity to MR, cortisone, and 11β-dehydrocorticosterone, respectively3, 4).
Types I and II 11β-HSD have been identified. Between them, type II 11β-hydroxysteroid dehydrogenase (11β-HSD II) is co-localized with MR in the kidney5), and its inhibition enhances sodium reabsorption in the distal nephron6). A congenital deficiency of 11β-HSD II results in a syndrome of apparent mineralocorticoid excess7). Mice lacking 11β-HSD II are hypertensive, along with marked hypertrophy and hyperplasia of the epithelium of the distal tubule in the kidney8).
However, the pathophysiological significance of the altered 11β-HSD activity has not been established in various hypertensive models. In essential hypertension, the hypertension has been in part attributed to deficient inactivation of cortisol by 11β-HSD9). Licorice and carbenoxolone may inhibit the activity of 11β-HSD II, allowing glucocorticoids to bind to MR and resulting in sodium retention and hypertension10, 11). In spontaneously hypertensive rats (SHR), one study showed an increase of 11β-HSD activity in the colon and renal medulla12), another study failed to demonstrate alterations of its activity in the kidney13).
The present study was aimed at investigating the regulation of renal 11β-HSD II in various rat models of hypertension. The mRNA level of 11β-HSD II was determined in the kidney of two-kidney, one-clip (2K1C), deoxycorticosterone acetate (DOCA)/salt, and NG-nitro-L-arginine methyl ester (L-NAME)-induced hypertension.
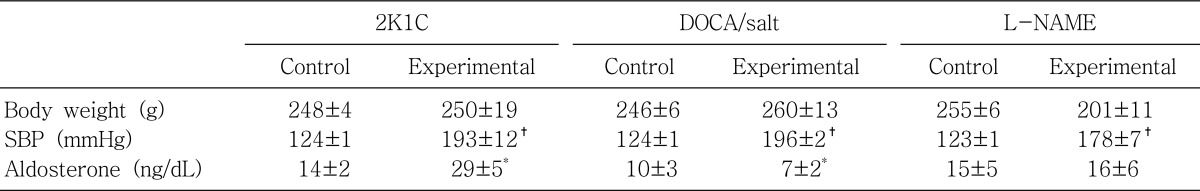
Male Sprague-Dawley rats weighing 150-200 g were used. Rats were kept in accordance with Institutional Guidelines of Experimental Animal Care and Use. Two-kidney, one-clip hypertension was made by constricting the left renal artery with a silver clip having an internal gap of 0.25 mm. Its control group was sham-operated without clipping the renal artery. Deoxycorticosterone acetate/salt hypertension was induced by subcutaneous implantation of silicone rubber containing DOCA (200 mg/kg), one week after unilateral nephrectomy. The rats were then supplied with 1.0% saline to drink. Its control group was also unilaterally nephrectomized and supplied with 1.0% saline to drink, but was without DOCA implantation. The third model of hypertension was made by treatment with L-NAME (100 mg/L drinking water). The control group was supplied with tap water without L-NAME.
They were kept for 4 weeks after inducing the hypertension. Systolic blood pressure was measured indirectly by tail-cuff method on the day of experiment. The trunk blood was collected by decapitation in a conscious state and then centrifuged at 1,500×g for 5 min. The plasma concentration of aldosterone was measured using a commercial radioimmunoassay kit (Diagnostic Products; Los Angeles, CA, USA).
After obtaining the trunk blood, the kidneys were rapidly removed. Total RNA was isolated using RNAzol solution according to the single-step method of Chomczynski and Sacchi14). RNAzol solution contained 0.025 M sodium citrate, 0.5% sarcosyl, 4.0 M guanidium thiocyanate, 0.1 M 2-mercaptoethanol, 0.2 M NaOAc, and phenol (pH 5.0). The concentration of total RNA was determined by the absorbance read at 260 nm. The integrity of RNA sample was confirmed through denaturing agarose gel electrophoresis.
For Northern blotting, RNA samples were resolved by electrophoresis through 8% formaldehyde-1.4% agarose denaturing gel buffered with 20 mM 3-N-morpholinopropane sulfonic acid and 1 mM EDTA (pH 7.4). The RNAs were then transferred overnight from gels to Nylon membrane (MSI; Westboro, MA, USA) using 20×SSC (1×SSC contained 0.15 mol/L NaCl and 0.015 mol/L sodium citrate). The membrane was cross-linked by exposure to UV light for approximately 2 min.
For the determination of 11β-HSD II mRNA levels, DNA probe was prepared using DNA polymerase I (Klenow) in the presence of α-32P-dCTP, dNTP mix, and denatured DNA template (25 ng) with Prime-a Gene® random prime labeling system (Promega; Madison, WI, USA). The membrane was prehybridized in a shaking water bath at 42℃ for 4 h in a solution containing 50% deionized formamide, 4×SSC, 100 µg/mL sheared and denatured salmon sperm DNA, 4×Denhardts solution, 0.1% sodium dodecyl sulfate (SDS), and 50 mM sodium phosphate (pH 6.5). The membrane was then hybridized at 42℃ for 16-20 h in the above solution containing approximately 1×106 cpm/µL of 32P-labeled 11β-HSD II cDNA. The rat 11β-HSD II cDNA was kindly provided by Dr. Gomez-Sanchez from Truman Memorial Veterans Hospital (Columbia, SC, USA)15). After hybridization, the membrane was washed four times for 15 min each at room temperature in washing solution I (2×SSC and 0.1% SDS), and then three times for 45 min each at 42℃ in washing solution II (0.1×SSC and 0.1% SDS). Drugs used were purchased from Sigma Chemical Company (St. Louis, MO, USA), unless stated otherwise. The membrane was then dried under the lamp for 30 min and exposed to X-ray films (O-MAT™, Kodak; Rochester, NY, USA). The mRNA levels were determined by densitometry and normalized with 18S rRNA, using a transmitter scanning videodensitometer (Zenith Video Densitometer, Biomed Instruments; Indianapolis, IN, USA).
Our study demonstrated an altered regulation of 11β-HSD II in the three models of secondary hypertension. The mRNA expression of 11β-HSD II was decreased in the clipped kidney of 2K1C hypertension and in the kidneys of L-NAME hypertension. On the contrary, the expression was increased in the non-clipped kidney of 2K1C hypertension and in the remnant kidney of DOCA/salt hypertension.
It has been shown that the activity and expression of 11β-HSD II are decreased in the kidney in Dahl salt-sensitive hypertension and Lyon hypertension16, 17). Furthermore, 11β-HSD II (-/-) mice demonstrate major features of the syndrome of apparent mineralocorticoid excess, along with striking hypertrophy and hyperplasia of the epithelium of the distal tubule8). It is likely that the down-regulation of 11β-HSD II has increased the binding of glucocorticoids to MR, resulting in sodium retention and development of high blood pressure in 2K1C and L-NAME hypertension. On the other hand, it has been found that nephrectomy itself does not affect the expression of 11β-HSD II in the remnant kidney18). Therefore, the up-regulation of 11β-HSD II in DOCA/salt hypertension may play a compensatory role to dissipate the volume expansion and hypertension.
It has been well known that plasma renin activity and aldosterone concentrations are increased in 2K1C hypertension and suppressed in DOCA/salt hypertension. The present study also showed that the plasma level of aldosterone was increased in 2K1C hypertension, and decreased in DOCA/salt hypertension. On the contrary, it was not significantly altered in LNAME hypertension. In the presence of aldosterone in vitro, the catalytic activity of 11β-HSD II is increased in isolated renal cortical collecting ducts19). In this context, the up-regulation of 11β-HSD II in the non-clipped kidney of 2K1C hypertension may reflect increased activity of the renin-angiotensin-aldosterone axis. The amount of corticosterone going through the 11β-HSD II selectivity filter is likely to be reduced by aldosterone, reinforcing the occupancy of MR. Though having not been completely understood, there exists an interaction between the renin-angiotensin-aldosterone axis and 11β-HSD II20).
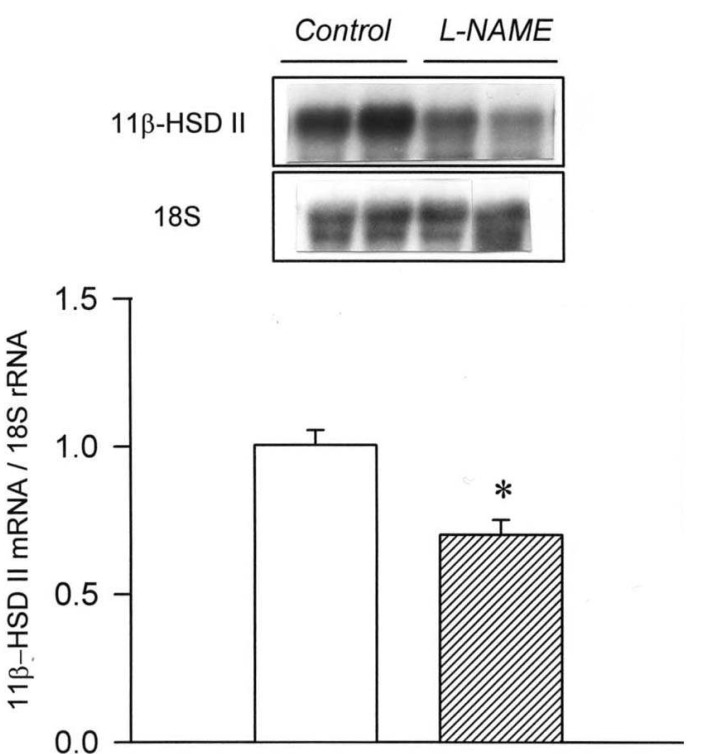
Administration L-NAME has been found to increase the plasma level of adrenocorticotropic hormone (ACTH)21). It was also demonstrated that ACTH dose-dependently decreased the expression and the enzyme activity of 11β-HSD II in human aortic endothelial cells22). The down-regulation of 11β-HSD II in the kidney may also in part be attributed to the release of ACTH in L-NAME hypertension in the present study. The renal expression of nitric oxide (NO) synthases was decreased in ACTH- and corticosterone-induced hypertension23). It has been well known that NO inhibits sodium reabsorption in the cortical collecting duct24). Taken together, L-NAME-induced hypertension may result from an interplay among NO, ACTH and 11β-HSD II.
In summary, our study demonstrates an altered regulation of 11β-HSD II in the kidney in 2K1C, DOCA/salt, and L-NAME-induced hypertension. The down-regulation of 11β-HSD II may contribute to the sodium retention and subsequently to the elevation of blood pressure in 2K1C and L-NAME hypertension, whereas the up-regulation may play a compensatory role to dissipate the sodium retention in DOCA/salt hypertension.
Acknowledgements
This work was supported by the research grant from the Korea Science and Engineering Foundation (97-0403-0901-3).
References
1. Eaton DC, Becchetti A, Ma H, Ling BN. Renal sodium channels: regulation and single channel properties. Kidney Int. 1995; 48:941–949. PMID: 8569103.

2. Renard S, Voilley N, Bassilana F, Lazdunski M, Barbry P. Localization and regulation by steroids of the alpha, beta and gamma subunits of the amiloride-sensitive Na+ channel in colon, lung and kidney. Pflugers Arch. 1995; 430:299–307. PMID: 7491252.
3. Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988; 242:583–585. PMID: 2845584.

4. Edwards CRW, Stewart PM, Burt D, Brett L, McIntyre MA, Sutanto WS, DeKloet ER, Monder C. Localization of 11β-hydroxysteroid dehydrogenase-tissue specific protector of the mineralocorticoid receptor. Lancet. 1988; 2:986–989. PMID: 2902493.
5. Rundle SE, Funder JW, Lakshmi V, Monder C. The intrarenal localization of mineralocorticoid receptors and 11β-dehydrogenase: immunocytochemical studies. Endocrinology. 1989; 125:1700–1704. PMID: 2547593.

6. Bailey MA, Unwin RJ, Shirley DG. In vivo inhibition of renal 11beta-hydroxysteroid dehydrogenase in the rat stimulates collecting duct sodium reabsorption. Clin Sci(Lond). 2001; 101:195–198. PMID: 11473496.
7. Wilson RC, Krozowski ZS, Li K, Obeyesekere VR, Razzaghy-Azar M, Harbison MD, Wei JQ, Shackleton CH, Funder JW, New MI. A mutation in the HSD11β2 gene in a family with apparent mineralocorticoid excess. J Clin Endocrinol Metab. 1995; 80:2263–2266. PMID: 7608290.
8. Kotelevtsev Y, Brown RW, Fleming S, Kenyon C, Edwards CR, Seckl JR, Mullins JJ. Hypertension in mice lacking 11beta-hydroxysteroid dehydrogenase type 2. J Clin Invest. 1999; 103:683–689. PMID: 10074485.
9. Walker BR, Stewart PM, Shackleton CHL, Padfield PL, Edwards CRW. Deficient inactivation of cortisol by 11 beta-hydroxysteroid dehydrogenase in essential hypertention. Clin Endocrinol(Oxf). 1993; 39:221–227. PMID: 8370136.
10. Stewart PM, Valentino R, Wallance AM, Burt D, Shackleton CHL, Edwards CRW. Mineralocorticoid activity of liquorice: 11β-hydroxysteroid dehydrogenase deficiency comes to age. Lancet. 1987; 2:821–824. PMID: 2889032.
11. Farese RV Jr, Biglieri EG, Shackleton CH, Irony I, Gomez-Fontes R. Licorice-induced hypermineralocorticoidism. N Engl J Med. 1991; 325:1223–1227. PMID: 1922210.

12. Pohlova I, Miksik I, Kunes J, Pacha J. 11beta-hydroxysteroid dehydrogenase activity in spontaneously hypertensive and Dahl rats. Am J Hypertens. 2000; 13:927–933. PMID: 10950402.
13. Hermans JJ, Steckel B, Thijssen HH, Janssen BJ, Netter KJ, Maser E. Comparison of 11 beta-hydroxysteroid dehydrogenase in spontaneously hypertensive and Wistar-Kyoto rats. Steroids. 1995; 60:773–779. PMID: 8585102.
14. Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987; 162:156–159. PMID: 2440339.

15. Zhou M-Y, Gomez-Sanchez EP, Cox DL, Cosby D, Gomez-Sanchez CE. Cloning, expression, and tissue distribution of the rat nicotinamide adenine dinucleotide-dependent 11β-hydroxysteroid dehydrogenase. Endocrinology. 1995; 136:3729–3734. PMID: 7649078.
16. Takeda Y, Inba S, Furukawa K, Miyamori I. Renal 11β-hydroxysteroid dehydrogenase in genetically saltsensitive hypertensive rats. Hypertension. 1998; 32:1077–1082. PMID: 9856977.

17. Lloyd-MacGilp SA, Nelson SM, Florin M, Lo M, McKinnell J, Sassard J, Kenyon CJ. 11β-hydroxysteroid dehydrogenase and corticosteroid action in Lyon hypertensive rats. Hypertension. 1999; 34:1123–1128. PMID: 10567193.

18. Escher G, Vogt B, Beck T, Guntern D, Frey BM, Frey FJ. Reduced 11beta-hydroxysteroid dehydrogenase activity in the remaining kidney following nephrectomy. Endocrinology. 1998; 139:1533–1539. PMID: 9528931.
19. Alfaidy N, Blot-Chabaud M, Bonvalet JP, Farman N. Vasopressin potentiates mineralocorticoid selectivity by stimulating 11 beta hydroxysteroid dehydrogenase in rat collecting duct. J Clin Invest. 1997; 100:2437–2442. PMID: 9366557.
20. Ricketts ML, Stewart PM. Regulation of 11beta-hydroxysteroid dehydrogenase type 2 by diuretics and the renin-angiotensin-aldosterone axis. Clin Sci(Lond). 1999; 96:669–675. PMID: 10334975.
21. Giordano M, Vermeulen EM, Trevani AS, Dran G, Andonegui G, Geffner JR. Nitric oxide synthase inhibitors enhance plasma levels of corticosterone and ACTH. Acta Physiol Scand. 1996; 157:259–264. PMID: 8800367.

22. Hatakeyama H, Inaba S, Taniguchi N, Miyamori I. Functional adrenocorticotropic hormone receptor in cultured human vascular endothelial cells: possible role in control of blood pressure. Hypertension. 2000; 36:862–865. PMID: 11082157.
23. Lou YK, Wen C, Li M, Adams DJ, Wang MX, Yang F, Morris BJ, Whitworth JA. Decreased renal expression of nitric oxide synthase isoforms in adrenocorticotropin-induced and corticosterone-induced hypertension. Hypertension. 2001; 37:1164–1170. PMID: 11304519.

24. Stoos BA, Garcia NH, Garvin JL. Nitric oxide inhibits sodium reabsorption in the isolated perfused cortical collecting duct. J Am Soc Nephrol. 1995; 6:89–94. PMID: 7579075.

Fig. 1
Representative Northern blot autoradiograms and densitometric data showing type II 11β-hydroxysteroid dehydrogenase (11β-HSD II) mRNA in the clipped kidney of two-kidney, one-clip rats. The open column represents the control and the hatched column depicts the hypertensive group (mean±SEM of 4 experiments each). *p<0.05, compared with control.
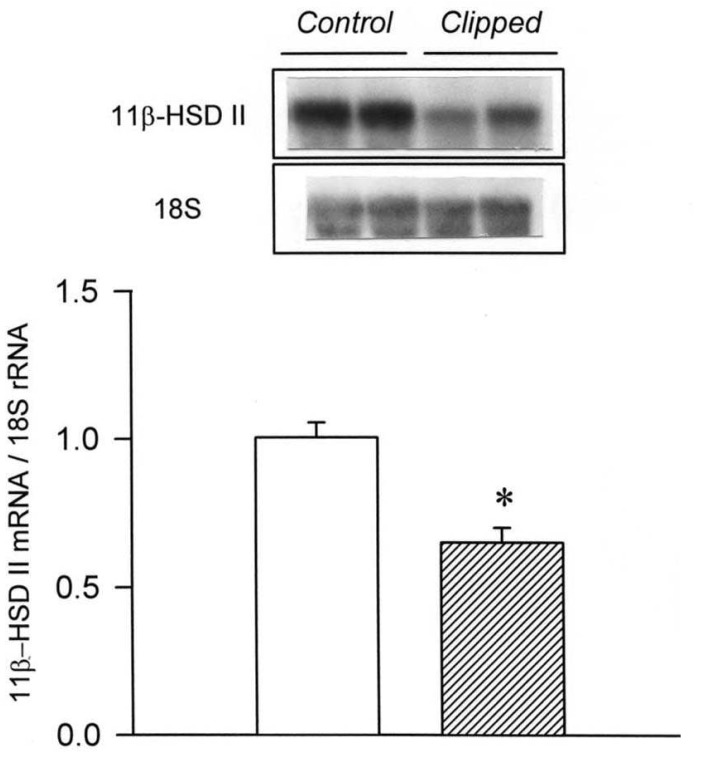
Fig. 2
Representative Northern blot autoradiograms and densitometric data showing 11β-HSD II mRNA in the non-clipped kidney of two-kidney, one-clip rats. The open column represents the control and the hatched column depicts the hypertensive group (mean±SEM of 4 experiments each). *p<0.05 compared with control.
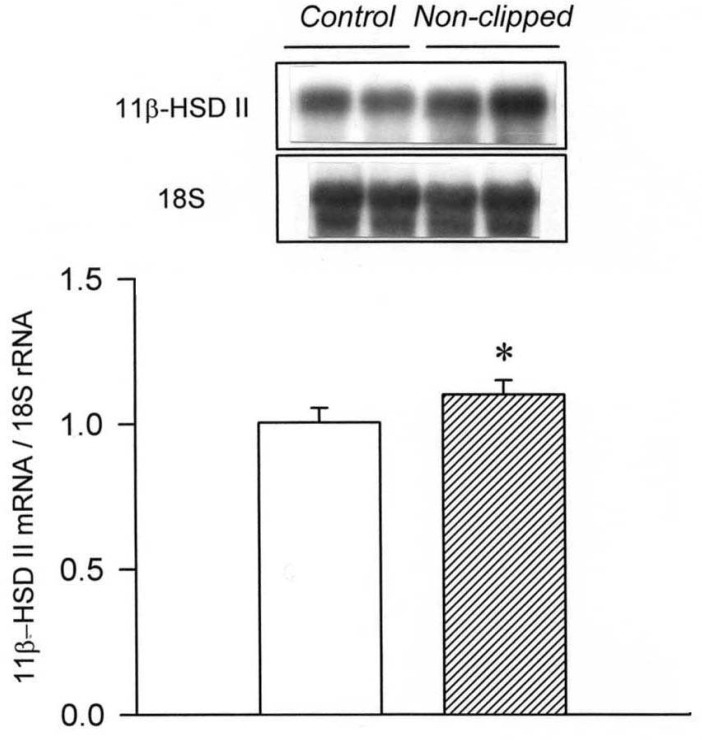
Fig. 3
Representative Northern blot autoradiograms and densitometric data showing type II 11β-hydroxysteroid dehydrogenase (11β-HSD II) mRNA in the remnant kidney of deoxycorticosterone acetate (DOCA)/salt hypertension.. The open column represents the control and the hatched column depicts the hypertensive group (mean±SEM of 4 experiments each). *p<0.05 compared with control.
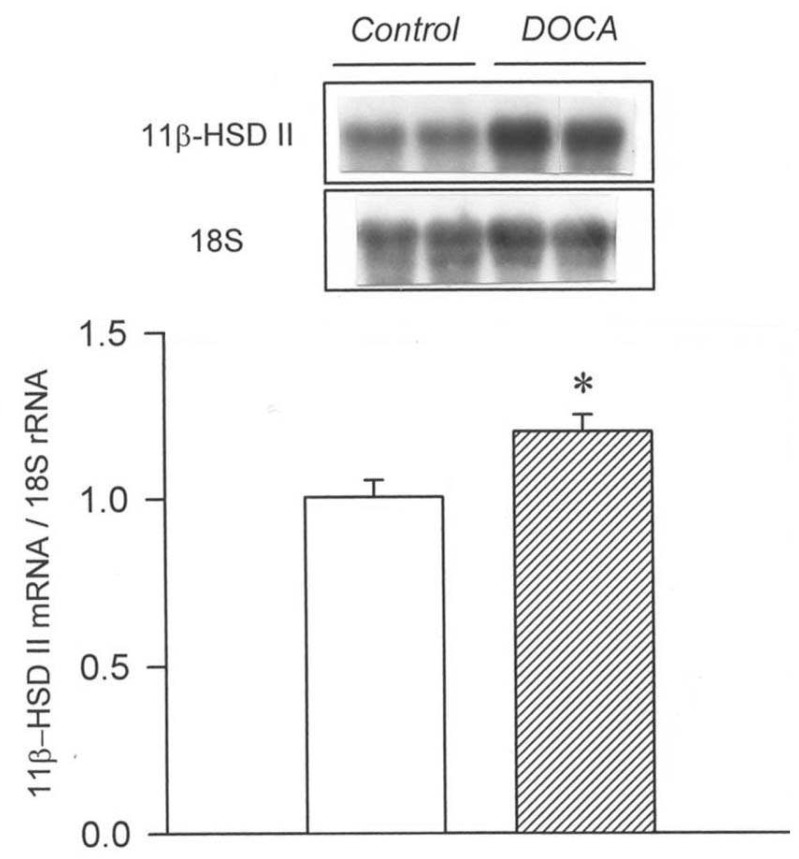
Fig. 4
Representative Northern blot autoradiograms and densitometric data showing type II 11β-hydroxysteroid dehydrogenase (11β-HSD II) mRNA in the kidney of NGnitro-L-arginine methyl ester (L-NAME) induced hypertension.. The open column represents the control and the hatched column depicts the hypertensive group (mean±SEM of 4 experiments each). *p<0.05 compared with control.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download