1. Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986; 233:305–312. PMID:
3014651.

2. Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992; 258:607–614. PMID:
1411571.

3. Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995; 9:484–496. PMID:
7737456.

4. Bertorello AM, Aperia A, Walaas SI, Nairn AC, Greengard P. Phosphorylation of the catalytic subunit of Na+, K(+)-ATPase inhibits the activity of the enzyme. Proc Natl Acad Sci U S A. 1991; 88:11359–11362. PMID:
1662394.
5. Nowicki S, Kruse MS, Brismar H, Aperia A. Dopamine-induced translocation of protein kinase C isoforms visualized in renal epithelial cells. Am J Physiol Cell Physiol. 2000; 279:C1812–C1818. PMID:
11078696.

6. Middleton JP, Khan WA, Collinsworth G, Hannun YA, Medford RM. Heterogeneity of protein kinase C-mediated rapid regulation of Na/K-ATPase in kidney epithelial cells. J Biol Chem. 1993; 268:15958–15964. PMID:
8393456.

7. Horie S, Moe O, Miller RT, Alpern RJ. Long-term activation of protein kinase c causes chronic Na/H antiporter stimulation in cultured proximal tubule cells. J Clin Invest. 1992; 89:365–372. PMID:
1310692.

8. Ruiz OS, Arruda JA. Regulation of the renal NaHCO
3 cotransporter by cAMP and Ca-dependent protein kinases. Am J Physiol. 1992; 262:F560–F565. PMID:
1314505.
9. Kosaka Y, Ogita K, Ase K, Nomura H, Kikkawa U, Nishizuka Y. The heterogeneity of protein kinase C in various rat tissues. Biochem Biophys Res Commun. 1988; 151:973–981. PMID:
3355565.

10. Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, Nishizuka Y. The structure, expression, and properties of additional members of the protein kinase C family. J Biol Chem. 1988; 263:6927–6932. PMID:
2834397.

11. Wetsel WC, Khan WA, Merchenthaler I, Rivera H, Halpern AE, Phung HM, Negro-Vilar A, Hannun YA. Tissue and cellular distribution of the extended family of protein kinase C isoenzymes. J Cell Biol. 1992; 117:121–133. PMID:
1556149.

12. La Porta CA, Comolli R. Biochemical and immunological characterization of calcium-dependent and -independent PKC isoenzymes in renal ischemia. Biochem Biophys Res Commun. 1993; 191:1124–1130. PMID:
8466490.
13. Aristimuño PC, Good DW. PKC isoforms in rat medullary thick ascending limb: selective activation of the delta-isoform by PGE2. Am J Physiol. 1997; 272:F624–F631. PMID:
9176373.
14. Ostlund E, Mendez CF, Jacobsson G, Fryckstedt J, Meister B, Aperia A. Expression of protein kinase C isoforms in renal tissue. Kidney Int. 1995; 47:766–773. PMID:
7752575.
15. Serlachius E, Svennilson J, Schalling M, Aperia A. Protein kinase C in the developing kidney: isoform expression and effects of ceramide and PKC inhibitors. Kidney Int. 1997; 52:901–910. PMID:
9328928.

16. Redling S, Pfaff IL, Leitges M, Vallon V. Immunolocalization of protein kinase C isoenzymes alpha, beta I, beta II, delta, and epsilon in mouse kidney. Am J Physiol Renal Physiol. 2004; 287:F289–F298. PMID:
15039141.
17. Hashimoto T, Ase K, Sawamura S, Kikkawa U, Saito N, Tanaka C, Nishizuka Y. Postnatal development of a brain-specific subspecies of protein kinase C in rat. J Neurosci. 1988; 8:1678–1683. PMID:
3367216.

18. Hirata M, Saito N, Kono M, Tanaka C. Differential expression of the beta I- and beta II-PKC subspecies in the postnatal developing rat brain; an immunocytochemical study. Brain Res Dev Brain Res. 1991; 62:229–238.
19. Pucéat M, Hilal-Dandan R, Strulovici B, Brunton LL, Brown JH. Differential regulation of protein kinase C isoforms in isolated neonatal and adult rat cardiomyocytes. J Biol Chem. 1994; 269:16938–16944. PMID:
8207017.

20. Rybin VO, Steinberg SF. Protein kinase C isoform expression and regulation in the developing rat heart. Circ Res. 1994; 74:299–309. PMID:
8293569.

21. Housey GM, Johnson MD, Hsiao WL, O'Brian CA, Murphy JP, Kirschmeier P, Weinstein IB. Overproduction of protein kinase C causes disordered growth control in rat fibroblasts. Cell. 1988; 52:343–354. PMID:
3345563.
22. Dong L, Stevens JL, Fabbro D, Jaken S. Regulation of protein kinase C isozymes in kidney regeneration. Cancer Res. 1993; 53:4542–4549. PMID:
8402625.
23. Mischak H, Goodnight JA, Kolch W, Martiny-Baron G, Schaechtle C, Kazanietz MG, Blumberg PM, Pierce JH, Mushinski JF. Overexpression of protein kinase C-delta and -epsilon in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem. 1993; 268:6090–6096. PMID:
8454583.

24. Berra E, Diaz-Meco MT, Dominguez I, Municio MM, Sanz L, Lozano J, Chapkin RS, Moscat J. Protein kinase C zeta isoform is critical for mitogenic signal transduction. Cell. 1993; 74:555–563. PMID:
7688666.
25. Szallasi Z, Kosa K, Smith CB, Dlugosz AA, Williams EK, Yuspa SH, Blumberg PM. Differential regulation by anti-tumor-promoting 12-deoxyphorbol-13-phenylacetate reveals distinct roles of the classical and novel protein kinase C isozymes in biological responses of primary mouse keratinocytes. Mol Pharmacol. 1995; 47:258–265. PMID:
7870033.
26. Pfaff IL, Wagner HJ, Vallon V. Immunolocalization of protein kinase C isoenzymes alpha, beta1 and betaII in rat kidney. J Am Soc Nephrol. 1999; 10:1861–1873. PMID:
10477137.
27. Saxena R, Saksa BA, Hawkins KS, Ganz MB. Protein kinase C beta I and beta II are differentially expressed in the developing glomerulus. FASEB J. 1994; 8:646–653. PMID:
8005392.
28. Dong LQ, Stevens JL, Jaken S. Biochemical and immunological characterization of renal protein kinase C. Am J Physiol. 1991; 261:F679–F687. PMID:
1928379.

29. Fukuzaki A, Kaneto H, Ikeda S, Orikasa S. Characterization of protein kinase C expression in human kidney. Tohoku J Exp Med. 1996; 178:263–269. PMID:
8727708.

30. Madsen KM, Tisher CC. Structural-functional relationships along the distal nephron. Am J Physiol. 1986; 250:F1–F15.

31. Kim WY, Jung JH, Park EY, Yang CW, Kim H, Nielsen S, Madsen KM, Kim J. Expression of protein kinase C isoenzymes alpha, betaI, and delta in subtypes of intercalated cells of mouse kidney. Am J Physiol Renal Physiol. 2006; 291:F1052–F1060. PMID:
16735462.
32. Madsen KM, Verlander JW, Kim J, Tisher CC. Morphological adaptation of the collecting duct to acidbase disturbances. Kidney Int. 1991; 40(Suppl 33):S57–S63.
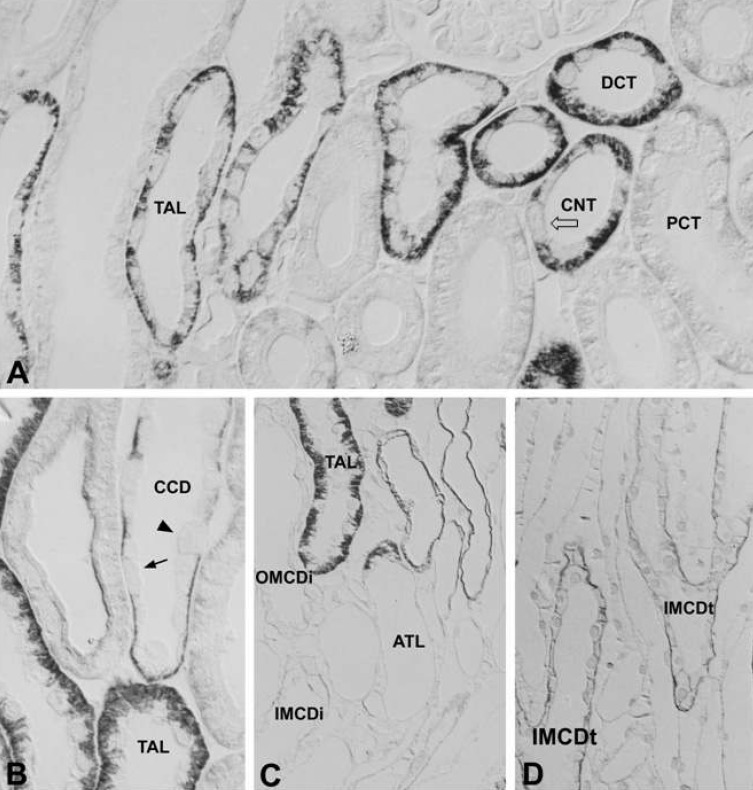
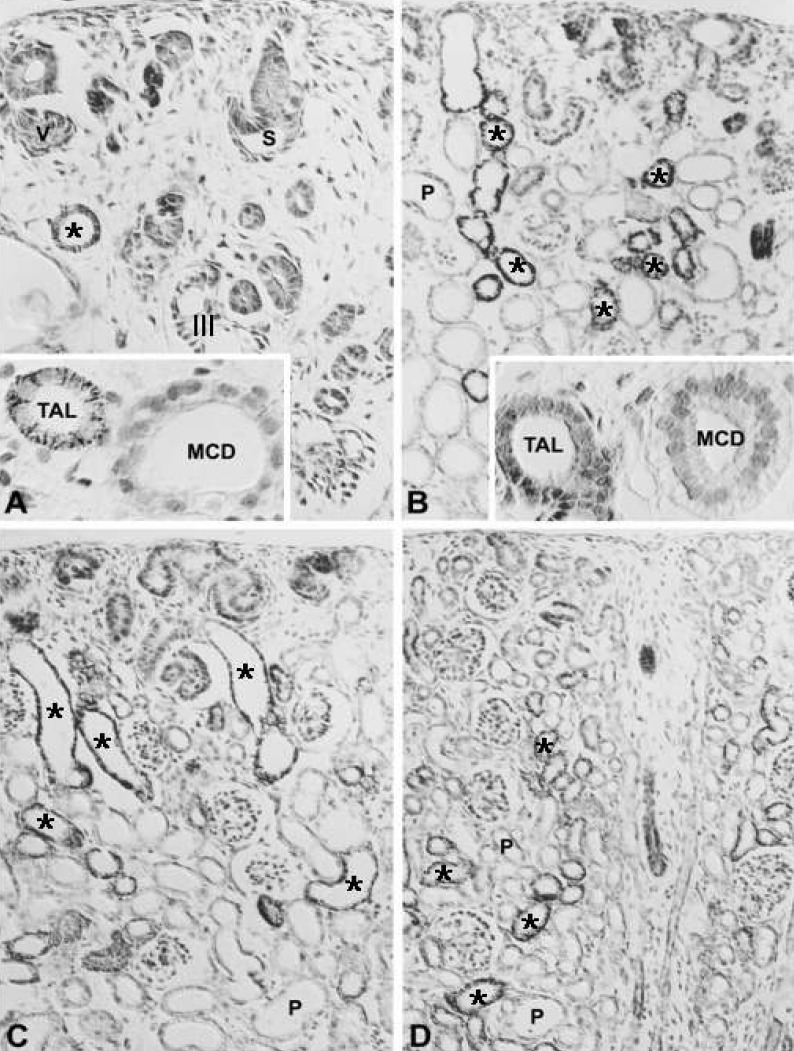
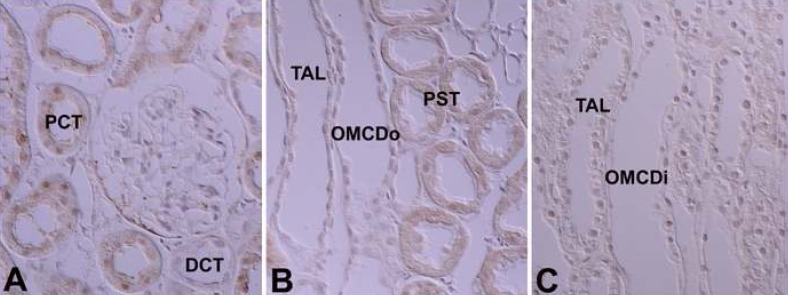
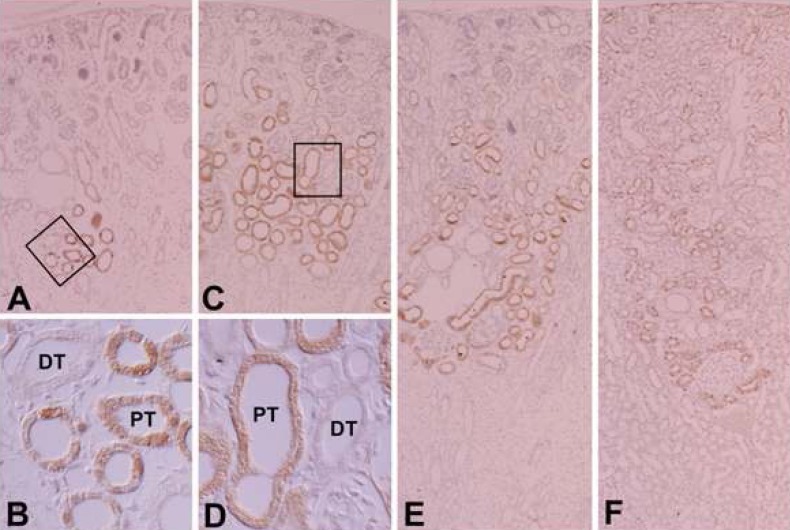




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download