Abstract
Metabolic acidosis was shown to correlate with deterioration of renal function in patients with rhabdomyolysis. The present study was aimed to investigate whether the changes of type 3 Na+/H+ exchanger (NHE3), type 1 Na+/HCO3- cotransporter (NBC1), and Na+,K+-ATPase α1 subunit may play a role in the pathogenesis of metabolic acidosis in glycerol-induced experimental rhabdomyolysis. Male Sprague-Dawley rats were deprived of fluid intake for 24 hours, and then were injected with 50% glycerol in normal saline (10 mL/kg, intramuscularly). At 24 hours after the glycerol injection, rats were sacrificed by decapitation. Control rats were injected with normal saline. The protein expression of NHE3, NBC1 and Na+,K+-ATPase α1 subunit was determined in the cortex of the kidney by immunoblotting and immunohistochemistry. Following the treatment of glycerol, creatinine clearance was significantly decreased, and high anion gap metabolic acidosis developed. In the experimental group, the expression of Na+,K+-ATPase α1 subunit was significantly decreased in the cortex of the kidney. On the contrary, the expression of NHE3 and NBC1 was significantly increased. Immunohistochemical analyses confirmed the immunoblotting data. In conclusion, the coordinate up-regulation of NHE3 and NBC1 may play an adaptive role against the metabolic acidosis in glycerol-induced rhabdomyolysis.
Rhabdomyolysis is a syndrome characterized by muscle necrosis and the release of intracellular muscle constituents into the circulation. The severity of illness ranges from asymptomatic elevations of muscle enzymes in the serum to life-threatening cases associated with extreme enzyme elevations, electrolyte imbalances, and acute kidney injury1). The occurrence of acute kidney injury (AKI) following untreated rhabdomyolysis has been put at between 17 and 33% of cases and accounts for 3 to 15% of all cases of AKI2). The animal model of experimental rhabdomyolysis is produced by intramuscular injection of hypertonic glycerol, which results in AKI and tubular dysfunction3, 4). Previous report demonstrated increased fractional sodium excretion and decreased cortical Na+, K+-ATPase activities in experimental rhabdomyolysis5). Moreover, in glycerol-induced AKI, tubular injuries are most severe at cortical segments of the proximal tubules and less extensive changes were observed in the distal tubules5-7).
In the kidney, Na+, K+-ATPase is expressed along the entire length of the basolateral membrane of the renal tubule and actively pumps sodium from the cell into the interstitium to set up the electrochemical gradient to allow sodium to be reabsorbed. In the proximal tubule, the primary route for the apical sodium transport is via type 3 Na+/H+ exchanger (NHE3). The proximal tubule reabsorbs 70-80% of the filtered load of bicarbonate, where apically expressed NHE3 provides the major route for proton secretion and type 1 Na+/HCO3- cotransporter (NBC1) enables the exit of bicarbonate across the basolateral membrane8-10). Therefore, several acid-base disorders can be accounted for by dysregulation of NHE3 and NBC111-13).
Systemic acidosis was shown to correlate with deterioration of renal function in patients with rhabdomyolysis14). Thus we hypothesize that the changes of NHE3 and NBC1 activity may play a role in the pathogenesis of metabolic acidosis in experimental rhabdomyolysis. We examined 1) whether there are changes in the protein abundance of NHE3 and NBC1 in the renal cortex in experimental rhabdomyolysis; 2) whether there are changes in the protein abundance of Na+,K+-ATPase.
The experimental procedure conformed to the Institutional Guidelines for Experimental Animal Care and Use. Male Sprague-Dawley rats weighing 250 to 270 g were used. They were deprived of fluid intake for 24 hours, and were injected with 50% glycerol in normal saline (10 mL/kg, intramuscularly). Control rats were injected with normal saline. The rats were maintained individually in metabolic cages to collect urine samples. At 24 hours after the glycerol injection, they were sacrificed for semiquantitative immunoblotting and immunohistochemical studies. Rats were anesthetized with ketamine (50 mg/kg, intraperitoneally) and a large laparotomy was made. Blood was collected from the inferior vena cava and analyzed for Na+, creatinine, HCO3- and pH. The right kidney was rapidly removed and processed for immunoblotting as described below. The left kidney was fixed by retrograde perfusion as described below.
The renal cortex was dissected and homogenized at 3,000 rpm in a solution that contained 250 mM sucrose, 1 mM ethylenediaminetetraacetate, 0.1 mM phenylmethylsulfonyl fluoride, and 10 mM Tris-HCl buffer (pH 7.6). The homogenates were centrifuged at 1,000 g for 15 min at 4℃ to remove the whole cells, nuclei and mitochondria; the supernatant was then pipetted off and kept on ice. The protein concentrations were measured by the Bicinchonic Acid Assay Kit (BioRad, Hercules, CA, USA).
All the samples were adjusted with isolation solution to reach the same final protein concentrations and solubilized at 65℃ for 15 min in SDS-containing sample buffer, and then stored at -20℃. To confirm equal loading of protein, an initial gel was stained with Coomassie Blue. SDS-PAGE was performed on 6% polyacrylamide gels. The proteins were transferred by gel electrophoresis (BioRad Mini Protean II) onto nitrocellulose membranes (Hybond ECL, Amersham Pharmacia Biotech, Little Chalfont, UK). The blots were subsequently blocked with 5% milk in PBS-T (80 mM Na2HPO4, 20 mM NaH2PO4, 100 mM NaCl and 0.1% Tween 20, pH 7.5) for 1 hour and incubated overnight at 4℃ with anti-rabbit polyclonal antibodies against NHE3 (1:750; Alpha Diagnostic, San Antonio, TX, USA) and NBC1 (1:750; Alpha Diagnostic, San Antonio, TX, USA), or anti-mouse monoclonal antibody against Na+,K+-ATPase α1 subunit (1:2,500; Upstate Biotechnology, Lake Placid, NY, USA). The membranes were then incubated for 2 h with secondary horseradish peroxidase-labeled anti-rabbit or anti-mouse IgG (1:1,200) antibodies. The labeling was visualized using an enhanced chemiluminescence system (Amersham, Buckinghamshire, UK) and it was finally analyzed using an Image Reader (LAS-3000 Imaging System, Fuji Photo Film).
The kidneys were fixed by in vivo perfusion of the abdominal aorta with cold 3% paraformaldehyde in 0.1 M cacodylate buffer (pH 7.4) for 3 min. The tissue was dehydrated in graded ethanol and left overnight in xylene. After embedding in paraffin, tissue sections were made at 6 µm and mounted on gelatin-coated glass slides.
The sections were de-paraffinized with xylene and rehydrated with graded ethanol. Sections had endogenous peroxidase activity blocked with 0.5% H2O2 in absolute methanol for 10 min. In a microwave oven the sections were boiled in target retrieval solution (1 mmol/L Tris, pH 9.0, with 0.5 mM EGTA) for 10 min. After cooling, nonspecific binding was blocked with 50 mM NH4Cl in PBS for 30 min followed by 3×10 min with PBS blocking-buffer containing 1% BSA, 0.05% saponin and 0.2% gelatin. The sections were incubated with primary antibody (diluted in PBS with 0.1% BSA and 0.3% Triton-X-100) overnight at 4℃. The sections were washed 3 times at 10 minute intervals with PBS wash-buffer containing 0.1% BSA, 0.05% saponin and 0.2% gelatin and incubated with horseradish peroxidase conjugated secondary antibody for 1 hr at room temperature. After rinsing with PBS wash-buffer three times, the sites with antibody-antigen reaction were visualized with a brown chromogen produced within 10 min by incubation with 0.05% 3,3'-diaminobenzidine tetrachloride dissolved in distilled water with 0.1% H2O2. Mayer's hematoxylin was used for counterstaining and thereafter dehydration coverslips were mounted with hydrophobic medium. Microscopy was carried out using an Olympus light microscope (Olympus, Tokyo, Japan).
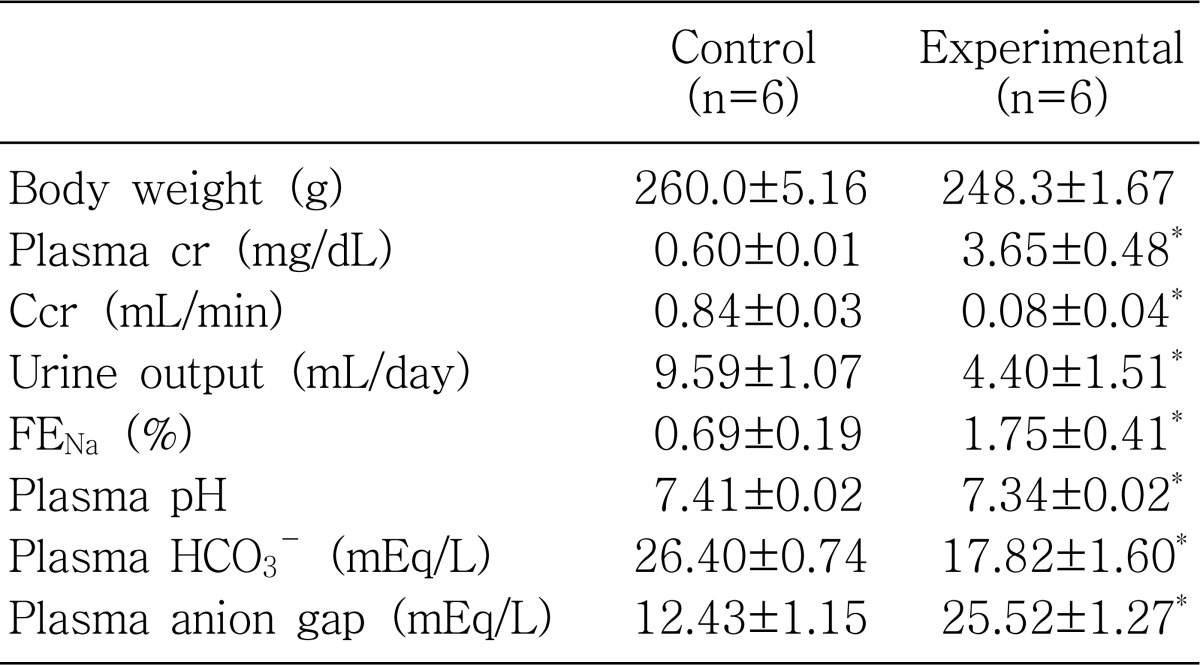
Table 1 shows the biochemical parameters. Glycerol-treated rats showed decreased creatinine clearance and increased plasma creatinine levels compared with that of controls. Accordingly, urine output was decreased. Fractional excretion of sodium was significantly increased, suggesting impaired tubular sodium reabsorption in glycerol-treated rats. Plasma pH and bicarbonate concentrations were decreased, and plasma anion gap was increased, suggesting the development of high anion gap metabolic acidosis.
Fig. 1 demonstrates the histological changes following treatment with glycerol. Tubular cell necrosis, intraluminal casts, hemorrhagic casts, tubular obstruction, and tubular dilatation, swelling and flattening of proximal tubular cells with brush border loss were present in glycerol-treated kidneys while glomerular morphology remained unchanged.
In the experimental group, the expression of Na+,K+-ATPase α1 subunit was significantly decreased in the cortex of the kidney (Fig. 2). However, the protein expression of NHE3 and NBC1 was significantly increased following the glycerol treatment (Fig. 3). Immunohistochemistry of NHE3 revealed apical membrane labeling of proximal tubule cells and thick ascending limb in the cortex and outer medulla, whereas the basolateral membranes were unlabeled. Consistent with the immunoblotting data, the NHE3 labeling was markedly increased in the experimental group (Fig. 4A, B). Immunolabeling of NBC1 appeared in the basolateral membrane of the proximal tubules, which was also more prominent in the experimental group (Fig. 4C, D).
In rhabdomyolysis, potentially toxic myocyte contents are released into the systemic circulation, and the renal consequences of this disturbance have been attributed to both intense vasoconstriction and renal tubular necrosis1). Myoglobinuria plays a key role in the pathophysiology of AKI both in clinical settings that are characterized by muscle tissue injury and in a widely used animal model of glycerol induced rhabdomyolysis1). In the present study, treatment with glycerol resulted in an oliguric renal failure associated with tubular necrosis in the proximal tubule. The serum creatinine level was increased along with a decrease of its renal clearance. Fractional excretion of sodium was increased, indicating impairment of tubular sodium reabsorption. In addition, plasma pH and bicarbonate concentrations were significantly decreased and plasma anion gap was increased, suggesting the development of high anion gap metabolic acidosis.
The tubular sodium reabsorption occurs through a two-step mechanism, i.e., primary active transport out of the cell exerted by Na+,K+-ATPase at the basolateral membrane, followed by a passive entry at the luminal membrane via NHE3 or other sodium cotransporters15, 16). Previous studies have demonstrated that glycerol injection results in proximal tubular injury and decreased renal cortical Na+,K+-ATPase activities5-7). The present study also revealed that the expression of α1 subunits of Na+,K+-ATPase was markedly decreased in association with tubular necrosis following treatment with glycerol. It is speculated that downregulation of Na+,K+-ATPase may be attributed to proximal tubular injury. It is noteworthy that the down-regulation of Na+,K+-ATPase may be causally related with an increased urinary excretion of sodium even though NHE3 abundance was increased. Thus it could be speculated that Na+,K+-ATPase activity overrules the NHE3-induced sodium reabsorption in experimental rhabdomyolysis, and may suggest that factors other than the acid-base state of the animals may have affected proximal tubule function.
Metabolic acidosis significantly increases urinary acid excretion, associated with a number of adaptive changes in renal tubules that contribute to increased urinary acidification. These adaptations may be attributed to an increased activity of Na+/H+ exchanger and bicarbonate reabsorptive capacity in the proximal tubules17). Na+/HCO3- cotransporter was initially localized by functional studies to the basolateral membrane of the proximal tubule, where it plays a role in mediating electrogenic HCO3- efflux18). In the present study, immunohistochemical analysis of the anti-NBC1 antibody also produced a strong and exclusively basolateral labeling in proximal tubules. These results are consistent with a view that NBC1 mediates basolateral proximal tubule HCO3- efflux19, 20). Our results revealed that the protein abundance of NBC1 and NHE3 in the proximal tubule was coordinately increased in response to metabolic acidosis induced by glycerol treatment, suggesting its functional up-regulation in response to metabolic acidosis. Taken together in vitro microperfusion studies, activities of the apically expressed NHE3 and basolaterally expressed NBC1 are up-regulated in response to metabolic acidosis11), these findings may suggest that systemic pH levels play a significant role in the regulation of HCO3- reabsorption in the proximal tubule.
In conclusion, the coordinate up-regulation of NHE3 and NBC1 may play an adaptive role against the metabolic acidosis in glycerol-induced rhabdomyolysis.
Acknowledgements
This work was supported by a grant from Chonnam National University Hospital Research Institute of Clinical Medicine (CUHRICM-U-2007018).
References
1. Vanholder R, Sever MS, Erek E, Lameire N. Rhabdomyolysis. J Am Soc Nephrol. 2000; 11:1553–1561. PMID: 10906171.

2. Beetham R. Biochemical investigation of suspected rhabdomyolysis. Ann Clin Biochem. 2000; 37:581–587. PMID: 11026512.

3. Paller MS. Hemoglobin- and myoglobin-induced acute renal failure in rats: role of iron in nephrotoxicity. Am J Physiol. 1988; 255:F539–F544. PMID: 3414810.

4. Shah SV, Walker PD. Evidence suggesting a role for hydroxyl radical in glycerol-induced acute renal failure. Am J Physiol. 1988; 255:F438–F443. PMID: 2843051.

5. Rodrigo R, Bosco C, Herrera P, Rivera G. Amelioration of myoglobinuric renal damage in rats by chronic exposure to flavonol-rich red wine. Nephrol Dial Transplant. 2004; 19:2237–2244. PMID: 15238628.

6. Singh D, Chander V, Chopra K. Carvedilol, an antihypertensive drug with antioxidant properties, protects against glycerol-induced acute renal failure. Am J Nephrol. 2003; 23:415–421. PMID: 14573997.

7. Nagano T, Mori-Kudo I, Tsuchida A, Kawamura T, Taiji M, Noguchi H. Ameliorative effect of hepatocyte growth factor on glycerol-induced acute renal failure with acute tubular necrosis. Nephron. 2002; 91:730–738. PMID: 12138279.

8. Mahnensmith RL, Aronson PS. The plasma membrane sodium-hydrogen exchanger and its role in physiological and pathophysiological processes. Circ Res. 1985; 56:773–788. PMID: 2988813.

9. Preisig PA, Ives HE, Cragoe EJ Jr, Alpern RJ, Rector FC Jr. Role of the Na+/H+ antiporter in rat proximal tubule bicarbonate absorption. J Clin Invest. 1987; 80:970–978. PMID: 2888788.
10. Soleimani M, Grassi SM, Aronson PS. Stoichiometry of Na+-HCO3- cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J Clin Invest. 1987; 79:1276–1280. PMID: 3558825.
11. Preisig PA, Alpern RJ. Chronic metabolic acidosis causes an adaptation in the apical membrane Na/H antiporter and basolateral membrane Na(HCO3)3 symporter in the rat proximal convoluted tubule. J Clin Invest. 1988; 82:1445–1453. PMID: 2844858.
12. Eladari D, Leviel F, Pezy F, Paillard M, Chambrey R. Rat proximal NHE3 adapts to chronic acid-base disorders but not to chronic changes in dietary NaCl intake. Am J Physiol Renal Physiol. 2002; 282:F835–F843. PMID: 11934693.
13. Amlal H, Chen Q, Greeley T, Pavelic L, Soleimani M. Coordinated down-regulation of NBC-1 and NHE-3 in sodium and bicarbonate loading. Kidney Int. 2001; 60:1824–1836. PMID: 11703600.

14. Muckart DJ, Moodley M, Naidu AG, Reddy AD, Meineke KR. Prediction of acute renal failure following softtissue injury using the venous bicarbonate concentration. J Trauma. 1992; 33:813–817. PMID: 1474620.

15. Katz AI, Doucet A, Morel F. Na-K-ATPase activity along the rabbit, rat, and mouse nephron. Am J Physiol. 1979; 237:F114–F120. PMID: 223456.

16. Besarab A, Silva P, Epstein FH. Multiple pumps for sodium reabsorption by the perfused kidney. Kidney Int. 1976; 10:147–153. PMID: 135114.

17. Kunau RT Jr, Hart JI, Walker KA. Effect of metabolic acidosis on proximal tubular total CO2 absorption. Am J Physiol. 1985; 249:F62–F68. PMID: 3925794.
18. Boron WF, Boulpaep EL. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral HCO3- transport. J Gen Physiol. 1983; 81:53–94. PMID: 6833997.
19. Abuladze N, Lee I, Newman D, Hwang J, Pushkin A, Kurtz I. Axial heterogeneity of sodium-bicarbonate cotransporter expression in the rabbit proximal tubule. Am J Physiol. 1998; 274:F628–F633. PMID: 9530281.
20. Schmitt BM, Biemesderfer D, Romero MF, Boulpaep EL, Boron WF. Immunolocalization of the electrogenic Na+-HCO3- cotransporter in mammalian and amphibian kidney. Am J Physiol. 1999; 276:F27–F38. PMID: 9887077.
Fig. 1
Hematoxylin and Eosin stained sections of kidneys. There is tubular cell necrosis, intraluminal casts, hemorrhagic casts, tubular obstruction, and tubular dilatation, swelling and flattening of proximal tubular cells with brush border loss in glycerol-treated kidneys while glomerular morphology remains unchanged. Magnification ×100.
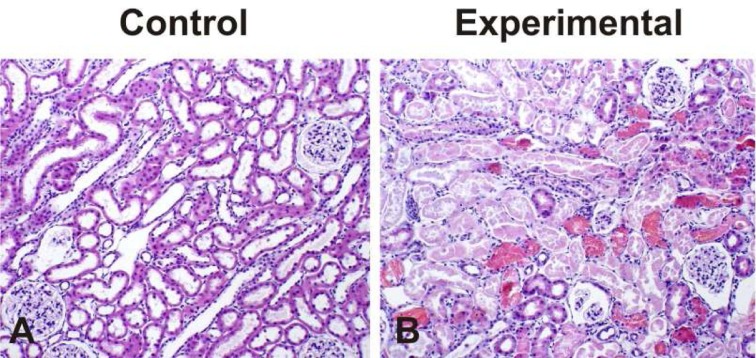
Fig. 2
Expression of Na+,K+-ATPase α1 subunit in the cortex of the kidney. The protein expression of Na+,K+-ATPase α1 subunit was significantly decreased in the cortex of the kidney following the glycerol treatment. *p<0.05 vs control rats. Magnification ×100.
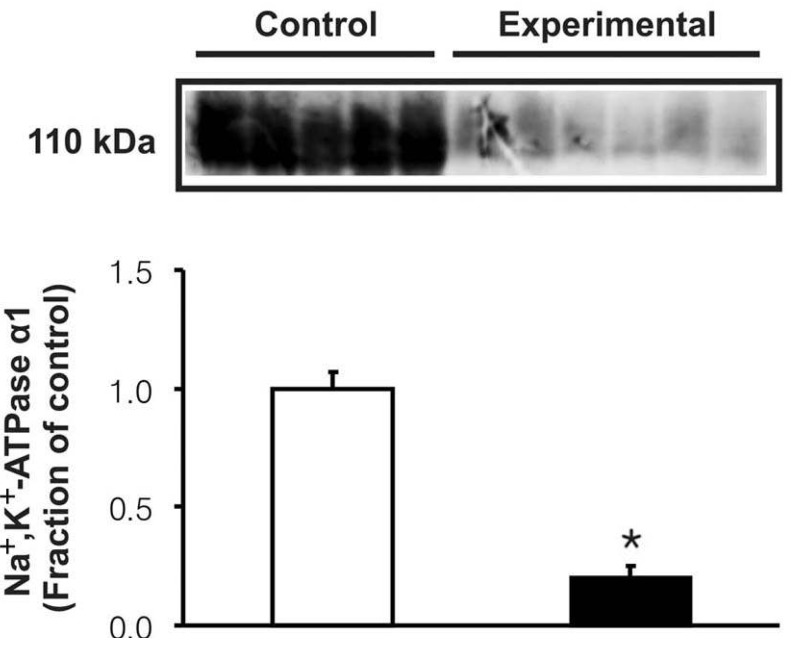
Fig. 3
Expression of type 3 Na+/H+ exchanger (NHE3) (A) and type 1 Na+/HCO3- cotransporter (NBC1) (B) in the cortex of the kidney. The protein expression of NHE3 and NBC1 was significantly increased following the glycerol treatment. *p<0.05 vs control rats.
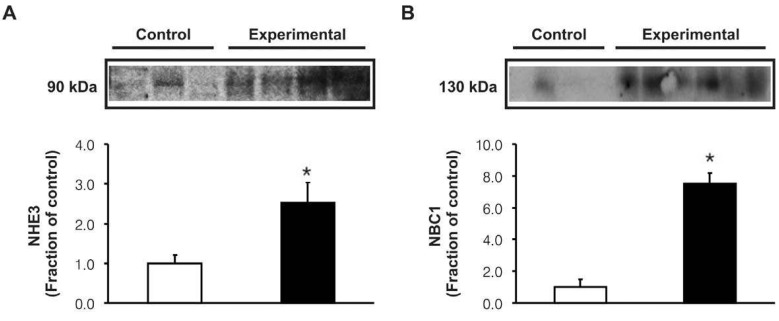
Fig. 4
Immunoperoxidase microscopy of type 3 Na+/H+ exchanger (NHE3) and type 1 Na+/HCO3- cotransporter (NBC1) in the cortex of the kidney. Immunohistochemistry of NHE3 revealed apical membrane labeling of proximal tubule (PT) cells in the cortex, whereas the basolateral membranes were unlabeled. Consistent with the immunoblotting data, the NHE3 labeling was markedly increased after the glycerol treatment (A and B). Immunolabeling of NBC1 appeared in the basolateral plasma membrane of PT cells, which was also more prominent in the experimental group (C and D). Magnification ×400.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download