Abstract
Metabolic acidosis has been considered as one of the reverse epidemiologic factors for the morbidity and mortality in maintenance hemodialysis patients (MHP). Expectedly, in the recent large scale epidemiologic study (The Dialysis Outcome Practice Pattern Study, DOPPS), a mild to moderate degree of predialysis metabolic acidosis has shown better nutritional status and lower relative risk for mortality and hospitalization in MHP. Similarly, another recent study of the largest sample size of MHP of more than 55,000 revealed the lowest unadjusted mortality with mild to moderate degree of predialysis HCO3 levels (17 to 23 mEq/L). However, it was reversed after case-mix and multivariate adjustment, including the malnutrition-inflammation complex syndrome, so that predialysis HCO3 levels of more than 22 mEq/L had a lower death risk. On view of this up-to-date on-going controversy about the optimal acid-base status for MHP, this paper will review the historical and break-through data about the pros and cons of metabolic acidosis published in the clinical human studies of MHP, a special subgroup of chronic kidney disease patients. Based on these results, if possible, we would like to suggest the best practice guideline, particularly, for the optimal predialysis HCO3 level, dialysate HCO3 concentration, and dietary protein intake.
As shown in animal and human studies of small groups, there has been somewhat of a consensus about the deleterious effect of metabolic acidosis, a common condition in chronic kidney disease (CKD), by engendering or worsening protein-energy malnutrition, inflammation, and bone diseases leading to a major role in increased mortality from short-term metabolic studies in CKD patients before the initiation of maintenance hemodialysis patients (MHP)1-5). However, predialysis metabolic acidosis in the majority of epidemiologic studies in MHP have shown an inverse correlation between a mild to moderate degree of hypobicarbonatemia and improved nutritional markers and also better survival than severe hypobicarbonatemia or hyperbicarbonatemia6-12). On the contrary, the most recent largest scale study of 56,385 MHP in all DaVita dialysis clinics across the United States led to opposite effects of metabolic acidosis in these MHP, depending on the analytical method13). In unadjusted data, the lowest mortality was associated with predialysis hypobicarbonatemia ranging from 17 to 23 mEq/L and progressively higher all-cause and cardiovascular mortality rates were associated with predialysis HCO3 levels of equal to or more than 23 mEq/L; whereas, after case-mix and malnutrition-inflammation complex syndrome (MICS) multivariate adjustment, this association was reversed, so that serum bicarbonate values >22 mEq/L had lower death risk13). Therefore, it is an appropriate time to review or revise how to define the adverse effects depending on the degree of metabolic acidosis in MHP in addition to its management, if necessary.
Even though the systemic consequence of metabolic acidosis arose from an increase in the total hydrogen ion concentration (pH) in the arterial blood measured by arterial blood gas analysis, it is not easily accessible for MHP in routine clinical practice. Therefore, blood total CO2 (or bicarbonate) levels of MHP are measured by an autoanalyzer and used as the indicator of the degree of metabolic acidosis. However, cautions are needed for the interpretation of serum chemistry measurements indicating metabolic acidosis due to spurious metabolic acidosis by several factors such as underfilling of sample tubes and shipping the blood samples for long distance by air transport leading to the evanescence of carbon dioxide before their measurements.
Generally, the severity of metabolic acidosis has shown an inverse correlation with the level of renal function, ie., the degree of metabolic acidosis became worse as renal function declines. Once maintenance hemodialysis has been established and bicarbonate stores have been repleted by delivery of bicarbonate, predialysis hypobicarbonatemia, theoretically, would be anticipated to return to normal values of 24 to 25 mEq/L. However, metabolic acidosis in MHP on conventional bicarbonate concentrations ranging from 33 to 38 mEq/L is still common, with one third to one-half of this population having predialysis serum bicarbonate levels below 22 mEq/L6).
The potential determinants of metabolic acidosis in MHP, have suggested to be associated with 1) endogenous acid production related to breakdown of protein intake and/or protein catabolic rate, 2) the administration of alkali by dialysis depending on the dialysis surface area and the transmembrane concentration gradient between bicarbonate level of dialysate and the blood, calcium containing or acid loading phosphate binders (sevelamer), and oral supplemental alkali source, 3) urinary acid excretion by residual renal function and loss of bicarbonate by stool, and 4) dilution of serum bicarbonate by interdialytic fluid gain. In a recent multivariate analysis of these factors, metabolic acidosis in MHP was associated more likely with increased protein nitrogen appearance (odds ratio [OR] 1.6 per 0.2 g/kg/day, p=0.001) and less likely with increased dialysis dose (OR 0.61 per 0.20 increase in Kt/V, p<0.001) and with increased calcium carbonate use (OR 0.38 per 2 g/day, p=0.003)14). Therefore, the key determinants of metabolic acidosis in MHP were suggested as the amount of protein intake and/or its catabolism, dialysis dose, and specific phosphate binders, rather than the degree of the administration of alkali by dialysis.
Experimental studies with animal models and clinical studies of humans with CKD before maintenance dialysis have shown that chronic metabolic acidosis has diverse potential adverse effects, ie., muscle wasting by increasing transcription gene of the ATP-dependent ubiquitin proteosome system and the enzyme branchedchain keto acid dehydrogenase (BCKAD) and reducing albumin synthesis with protein malnutrition inflammation complex syndrome, bone disease, impaired insulin sensitivity, beta-2 microglobulin accumulation, exacerbation of renal failure, impaired thyroid metabolism, stunted growth in children, cardiac disease with depressed myocardial contractility (though not proven), and increased inflammation15). Many CKD patients have progressive muscle wasting and malnutrition despite adequate dialysis, and it is now recognized that one of the major causes of malnutrition in CKD, particularly before the initiation of dialysis, is systemic metabolic acidosis16). In fact, the analysis of the Third National Health and Nutrition Examination Survey (NHANES III) showed that low serum bicarbonate was associated with hypoalbuminemia, explaining the high prevalence of hypoalbuminemia in CKD before dialysis17). Even in MHP, the correction of metabolic acidosis improved serum albumin levels of MHP4). However, it remains disputable whether the adverse nutritional effects and poor clinical outcomes of metabolic acidosis in CKD patients before initiation of hemodialysis can be applicable equally to MHP.
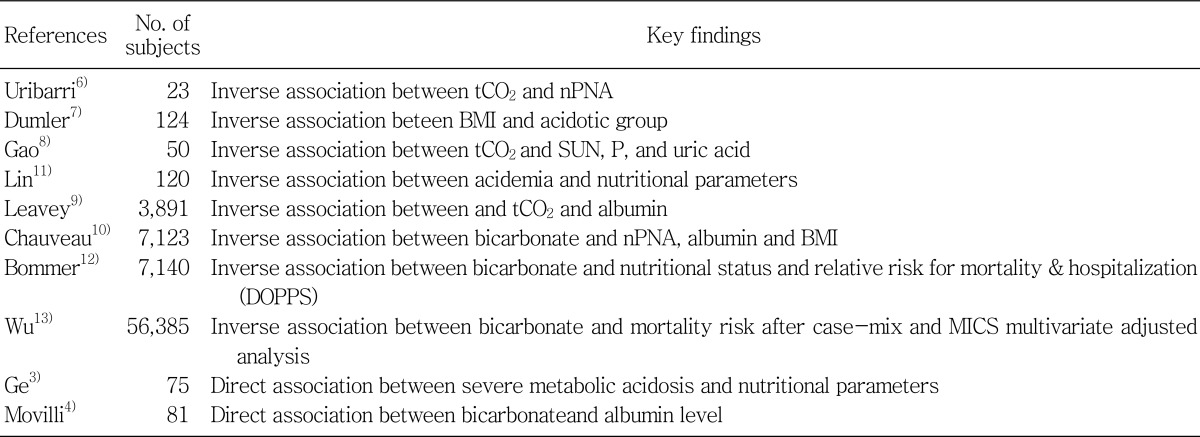
As shown in Table 1, the deleterious effect of metabolic acidosis on nutritional status has been noted in some earlier studies for small population of MHP by the studies of Ge et al and Movilli et al.3, 4), whereas, the vast majority of recent epidemiologic studies in MHP have revealed a paradoxically inverse relationship between nutritional parameters and metabolic acidosis. In the analysis of the HEMO study, Uribarri et al reported a significant inverse relationship between serum HCO3 and normalized protein equivalent of nitrogen appearance (nPNA) and showed that MHP with serum HCO3 levels (≤21 mEq/L), compared to those with serum HCO3 levels (≥25 mEq/L), had a higher serum creatinine and urea level6). Dumler et al reported a higher serum albumin, creatinine, and nPNA in MHP with metabolic acidosis7). Gao et al, Lin et al, and three epidemiologic studies with large sample sizes reported by Leavey et al, Chauveau et al, and the recent report by the Dialysis Outcomes and Practice Patterns Study (DOPPS, n=7,140) investigators also found an inverse association between serum HCO3 levels and nutritional factors, ie., moderate predialysis acidosis in levels between 19 and 22 mEq/L seemed to be associated with better nutritional status and lower relative risk for mortality or hospitalization than was observed in patients with normal ranges of midweek predialysis serum bicarbonate concentration of more than 24 mEq/L or severe acidosis less than 16 mEq/L8-12). In contradiction to most of the previous epidemiologic studies, the most recent study of the largest sample size by Wu et al. (n=56,835) showed significant associations between hypobicarbonatemia less than 23 mEq/L and higher death risk after case-mix and MICS multivariate adjustment13). Therefore, this study supports that MICS may be the substantial contributor to the counteracting associations between serum bicarbonate levels and mortality in MHD, which could be described as cardiovascular risk-factor paradox or reverse epidemiology in MHD as seen in other cardiovascular risk factors. Given this on-going controversy on epidemiologic studies, it still remains unresolved whether metabolic acidosis is truly harmful.
Based on the results of epidemiologic studies in contradiction to the adverse metabolic effects of metabolic acidosis and the lack of controlled studies examining the impact of various levels of hypobicarbonatemia, the complete normalization of blood bicarbonate levels in MHP is generally not recommended globally. The Kidney Disease Outcome Quality Initiatives (K/DOQI) and the European guidelines recommend serum bicarbonate level at or more than 22 mEq/L, but not to completely normal values.18, 19 Meanwhile, we admit that there is no clear consensus on the level of blood bicarbonate that should be targeted in MHP. Whatever the method to deliver or supplement bicarbonate to aim at normalization of serum bicarbonate levels is used, it might be associated with certain complications such as volume overload, exacerbation of hypertension, and potential exacerbation of vascular or soft tissue calcification.
Despite the conflicting results that have been shown in several studies between the association of predialysis acidosis and nutritional parameters such as serum albumin, nPNA, and BMI and those changes following the correction of predialysis metabolic acidosis, it is generally accepted that higher protein intake augments albumin synthesis; whereas, chronic metabolic acidosis has been shown to decrease albumin synthesis16). Therefore, a higher protein intake might increase albumin synthesis and this could outweigh the reduction in albumin synthesis due to metabolic acidosis. Furthermore, the subjective global assessment (SGA) correlates better with the outcome of renal failure patients than any other most sophisticated methods of nutritional assessment20). Similarly, in MHP, the subjective reported appetite was noted as a key indicator of general health and quality of life (QoL) as well as a main contributor to nutritional status and clinical outcome21). Accordingly, the protein-intake of more than 1.2 g/kg/day and highcalorie intake currently recommended by the National Kidney Foundation-K/DOQI Guidelines may be associated with benefits of survival through improved nutritional status, even though it would lead to moderate predialysis metabolic acidosis.
Though metabolic adverse effects by metabolic acidosis has been well established in animals and humans with CKD, particularly in short-term studies, most of the epidemiologic studies in MHP revealed inverse results showing that mild or moderate predialysis acidosis was associated with better nutritional status and lower relative risk for mortality or hospitalization, which could be similar to the reverse epidemiology as in cardiovascular risk factors in MHP. However, the MICS-adjusted associations between hypobicarbonatemia and mortality revealed the opposite direction in the most recent study with the largest sample size, which suggested that hypobicarbonatemia reflecting higher protein intake in MHP resulted in more prolonged survival in MICS-unadjusted data. Therefore, the foremost approaches to prevalent metabolic acidosis in MHD would be increased attention to intervening MICS with the improvement of malnutrition with adequate food intake, rather than the correction of serum bicarbonate levels, itself, by dialysate and/or by bicarbonate supplements. Until a uniform guideline of predialysis bicarbonate levels is available by more data to resolve our limited understanding of metabolic acidosis in MHP, its target level could be at or more than 22 mEq/L, but less than 24-25 mEq/L.
References
1. Papadoyannakis NJ, Stefanidis CJ, McGeown M. The effect of the correction of metabolic acidosis on nitrogen and potassium balance of patients with chronic renal failure. Am J Clin Nutr. 1984; 40:623–627. PMID: 6089541.

2. Kraut JA, Mishler DR, Singer FR, Goodman WG. The effects of metabolic acidosis on bone formation and bone resorption in the rat. Kidney Int. 1986; 30:694–700. PMID: 3784302.

3. Ge YQ, Wu ZL, Xu YZ, Liao LT. Study on nutritional status of maintenance hemodialysis patients. Clin Nephrol. 1998; 50:309–314. PMID: 9840319.
4. Movilli E, Zani R, Carli O, Sangalli L, Pola A, Camerini C, Cancarini GC, Scolari F, Feller P, Maiorca R. Correction of metabolic acidosis increases serum albumin concentrations and decreases kinetically evaluated protein intake in haemodialysis patients: a prospective study. Nephrol Dial Transplant. 1998; 13:1719–1722. PMID: 9681718.

5. Kraut JA, Kurtz I. Metabolic acidosis of CKD: diagnosis, clinical characteristics, and treatment. Am J Kidney Dis. 2005; 45:978–993. PMID: 15957126.

6. Uribarri J, Levin NW, Delmez J, Depner TA, Ornt D, Owen W, Yan G. Association of acidosis and nutritional parameters in hemodialysis patients. Am J Kidney Dis. 1999; 34:493–499. PMID: 10469860.

7. Dumler F, Falla P, Butler R, Wagner C, Francisco K. Impact of dialysis modality and acidosis on nutritional status. ASAIO J. 1999; 45:413–417. PMID: 10503617.

8. Gao H, Lew SQ, Bosch JP. Moderate metabolic acidosis and its effects on serum parameters in hemodialysis patients. Nephron. 2000; 86:135–138. PMID: 11014982.

9. Leavey SF, Strawderman RL, Young EW, Saran R, Roys E, Agodoa LY, Wolfe RA, Port FK. Cross-sectional and longitudinal predictors of serum albumin in hemodialysis patients. Kidney Int. 2000; 58:2119–2128. PMID: 11044233.

10. Chauveau P, Fouque D, Combe C, Laville M, Canaud B, Azar R, Cano N, Aparicio M, Leverve X. Acidosis and nutritional status in hemodialyzed patients. French Study Group for Nutrition in Dialysis. Semin Dial. 2000; 13:241–246. PMID: 10923352.
11. Lin SH, Lin YF, Chin HM, Wu CC. Must metabolic acidosis be associated with malnutrition in haemodialysed patients? Nephrol Dial Transplant. 2002; 17:2006–2010. PMID: 12401862.

12. Bommer J, Locatelli F, Satayathum S, Keen ML, Goodkin DA, Saito A, Akiba T, Port FK, Young EW. Association of predialysis serum bicarbonate levels with risk of mortality and hospitalization in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2004; 44:661–671. PMID: 15384017.

13. Wu DY, Shinaberger CS, Regidor DL, McAllister CJ, Kopple JD, Kalantar-Zadeh K. Association between Serum Bicarbonate and Death in Hemodialysis Patients: Is It Better to Be Acidotic or Alkalotic? Clin J Am Soc Nephrol. 2006; 1:70–78. PMID: 17699193.

14. Soudan K, Ricanati ES, Leon JB, Sehgal AR. Determinants of metabolic acidosis among hemodialysis patients. Hemodial Int. 2006; 10:209–214. PMID: 16623676.

15. Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996; 335:1897–1905. PMID: 8948566.
16. Mitch WE. Metabolic acidosis stimulates protein metabolism in uremia. Miner Electrolyte Metab. 1996; 22:62–65. PMID: 8676827.
17. Eustace JA, Astor B, Muntner PM, Ikizler TA, Coresh J. Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney Int. 2004; 65:1031–1040. PMID: 14871424.

18. Locatelli F, Fouque D, Heimburger O, Drueke TB, Cannata-Andia JB, Horl WH, Ritz E. Nutritional status in dialysis patients: a European consensus. Nephrol Dial Transplant. 2002; 17:563–572. PMID: 11917047.

19. National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003; 42(Suppl 3):S1–S201. PMID: 14520607.
20. Blumenkrantz MJ, Kopple JD, Gutman RA, Chan YK, Barbour GL, Roberts C, Shen FH, Gandhi VC, Tucker CT, Curtis FK, Coburn JW. Methods for assessing nutritional status of patients with renal failure. Am J Clin Nutr. 1980; 33:1567–1585. PMID: 7395778.

21. Kalantar-Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr. 2004; 80:299–307. PMID: 15277149.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download