Abstract
Urea accumulation in the renal inner medulla plays a key role in the maintenance of maximal urinary concentrating ability. Urea transport in the kidney is mediated by transporter proteins that include renal urea transporter (UT-A) and erythrocyte urea transporter (UT-B). UT-A1 and UT-A2 are produced from the same gene. There is an active tonicity-responsive enhancer (TonE) in the promoter of UT-A1, and the UT-A1 promoter is stimulated by hypertonicity via tonicity-responsive enhancer binding protein (TonEBP). The downregulation of UT-A2 raises the possibility that TonEBP also regulates its promoter. There is some evidence that TonEBP regulates expression of UT-A in vivo; (1) during the renal development of the urinary concentrating ability, expression of TonEBP precedes that of UT-A1; (2) in transgenic mice expressing a dominant negative form of TonEBP, expression of UT-A1 and UT-A2 is severely impaired; (3) in treatment with cyclosporine A, TonEBP was significantly downregulated after 28 days. This downregulation involves mRNA levels of UT-A2; (4) in hypokalemic animals, downregulation of TonEBP contributed to the down regulation of UT-A in the inner medulla. These data support that TonEBP directly contributes to the urinary concentration and renal urea recycling by the regulation of urea transporters.
Urea is an abundant solute in the mammalian renal medulla and the accumulation of urea is important for the generation of concentrated urine. The concentration of urea in the inner medulla is produced by the recycling of urea, secretion into the ascending thin limb (ATL) of the Henle's loop via the UT-A2 urea transporter, and reabsorption in the inner medullary collecting duct (IMCD) via UT-A1 urea transporter1). UT-A1 and UT-A2 are produced from the same gene. The promoter of UT-A1 is in the 5' flanking region while the promoter for UTA2 is in the intron 12. There is an active tonicity-responsive enhancer (TonE) in the promoter of UT-A1, and the UT-A1 promoter is stimulated by hypertonicity via TonEBP2). The downregulation of UT-A2 raises the possibility that tonicity-responsive enhancer binding protein (TonEBP) also regulates its promoter. On the other hand, severe atrophy of the renal medulla occurs in transgenic mice expressing a dominant negative TonEBP which may suggest that the downregulation of UT-A1 and UT-A2 is the consequence of the atrophy. A new animal model in which TonEBP can be specifically inhibited without morphological changes in the renal medulla is needed to directly test the role of TonEBP in the expression of UT-A in vivo. The physiological role of TonEBP in the renal medulla is summarized in Fig. 1. The signal to stimulate TonEBP comes from interstitial hypertonicity created by the primary sodium transporters, the sodium/chloride cotransporter (NCC) and sodium/potassium/chloride cotransporter (NKCC2). We propose that the NCC creates local hypertonicity in the cortex, especially in the medullary ray. This is consistent with expression of TonEBP in the cortical collecting ducts and the straight portion of the proximal tubule3).
In the developing rat kidney, UT-A first appears in the inner medullary colleting duct (IMCD) and in the developing long-loop descending thin limb (DTL) at the base of the renal papilla right after birth. As the UT-A-positive long-loop DTL descends into the renal papilla, UT-A immunoreactivity decreases and is barely detectable in animals after 3 weeks of age. In contrast, the expression of UT-A in short-loop DTL does not occur until 2 weeks after birth, the time they reach the ISOM, but the level of expression is very strong and remains increased in the adult kidney. UT-A immunoreactivity in the IMCD gradually increased after birth and reached adult levels when the animals were 3 weeks of age. UT-B immunoreactivity appears in descending vasa recta (DVR) shortly before birth and gradually increases during the first 2-3 weeks after birth. It is noteworthy that the UT-B-positive DVR do not form vascular bundles until the animals are 2 weeks old. In the developing kidney revealed that UT-B is expressed in the DVR already before birth and gradually increases during the first 2 wk after birth, indicating that countercurrent exchange between ascending vasa recta and DVR can occur already in the neonatal kidney. Interestingly, the formation of vascular bundles coincided with the appearance of UT- A2-positive short-loop DTL in the inner stripe of outer medulla and was followed by a striking increase in the expression of UT-A2 and UT-B with a clear delineation of the inner stripe of the outer medulla (ISOM) The expression of urea transporters in the neonatal rat renal medulla during the first 2 weeks after birth coincides with the development of the ability to produce a concentrated urine. The striking increase in UT-A2 and UT-B immunoreactivity 2 weeks after birth is closely associated with the descent of short-loop DTL from superficial nephrons and the formation of vascular bundles in the ISOM4).
TonEBP expression is detected as early as the fetal age of 16 days in endothelial cells surrounding the medullary collecing duct (MCD). Tubular expression is first detectable at the fetal age of 18 days. TonEBP expression increases steadily through birth until postnatal day 21, when the adult pattern of expression is established coincidentally with the full maturation of the renal medulla and urine-concentrating ability. During kidney development, TonEBP expression is largely limited to the medulla. In addition, the timing of TonEBP expression either precedes or coincides with that of its target genes. AR and UT-A are not expressed until postnatal day 1. The level of AR and UT-A expression rises slowly over the next 3 weeks in parallel with the expression of TonEBP. SMIT mRNA is expressed in the fetal kidney, but the level of expression parallels that of TonEBP through birth and the early postnatal stage. These data are consistent with the view that TonEBP stimulates expression of its target genes during development5).
In the developing kidney, TonEBP expression is largely limited to the medulla. In addition, the timing of TonEBP expression either precedes or coincides with that of its target genes. UT-As are not expressed until postnatal day 1. The level of UT-A expression rises slowly over the next 3 weeks in parallel with the expression of TonEBP. These data are consistent with the view that TonEBP stimulates expression of its target genes during development. If TonEBP is indeed driving the expression of UT-A in neonatal kidneys, it can be stated that hypertonicity is required for generation of the high urea concentrations that account for more than two-thirds of medullary osmolality during antidiuresis. In other words, TonEBP is an important part of the urine-concentrating mechanism in addition to its widely known role in protecting renal medullary cells from the deleterious effects of hyperosmolality. This can be tested directly once genetically modified mice with a deficiency in TonEBP expression in the kidney are available5).
In long-term cyclosporine A (CsA) administration, TonEBP is inhibited in the renal medulla in the form of reduced protein expression and cytoplasmic shift. This seems to be secondary to the reduced medullary tonicity as a result of downregulation of NKCC2 and Na-K-ATPase. The inhibition of TonEBP contributes to the defect in urinary concentration as a result of reduced transcription of its target genes, including UTA2. It is possible that there are more, yet-to-be-identified, target genes of TonEBP whose downregulation contributes to other aspects of nephrotoxicity that is induced by long-term CsA administration. Both the CsA- and vehicle-treated rats responded to dDAVP, although the urinary osmolality was significantly lower and the urinary flow was higher in the CsA-treated rats. In the vehicle-treated rats, infusion of dDAVP did not affect the expression of the sodium transporters except for a moderate, posttranscriptional decrease in the expression of NCC. The expression of TonEBP and its target genes did not change significantly despite a variety of changes in their transcript levels. The disconnection between levels of mRNA and proteins illustrates the complexity of regulation in response to long-term dDAVP administration. In the CsA-treated rats, infusion of dDAVP restored expression of NKCC2 in the outer medulla but not in the cortex. NCC and Na-K-ATPase were not affected by dDAVP. Of interest, there was posttranscriptional correction of TonEBP expression : Increased expression of TonEBP protein and nuclear localization without changes in transcript levels. These data suggest that the activity of NKCC2 in the outer medulla regulates TonEBP at the level of protein abundance and nuclear translocation in the renal medulla. As expected from the restoration of TonEBP expression and nuclear localization, transcript levels of the TonEBP target genes increased significantly, including UT-A1 and UT-A2. Expression of corresponding proteins showed a more complex pattern, indicating multiple layers of post-transcriptional regulation6).
In the developing mouse kidney, there was no UT-A immunoreactivity in the developing uriniferous tubules, including the collecting ducts up to F15. UT-A immunoreactivity appeared first on F16 in some of the developing DTLs. UT-A was detected in the terminal part of the AQP1-positive DTL, which was directly connected to the AQP1-negative TAL. By the P1, many DTLs express high levels of UT-A. In 4- and 7-day-old pups, UT-A immunoreactivity was seen in areas corresponding to the future medullary ray and outer medulla. From P14 onward, strong UT-A immunoreactivity was seen in the short-looped DTLs that formed bundles in the middle part of the inner stripe of the outer medulla. On the other hand, the intensity of UT-A immunoreactivity in the shorter long-looped DTLs, which were located at the site of the future innermost part of the inner stripe of the outer medulla, was markedly decreased. In developing MCDs, UT-A was first detected in the papilla on F18. The level of UT-A expression in the papilla increased dramatically after birth, showing the similar subcellular distribution seen in adults. Thus UT-A in the DTL is expressed a few days ahead of UT-A1 in the MCD. TonEBP immunoreactivity was first detected in the renal medulla on F15 and gradually increased afterward. At this stage, TonEBP was intense in the cytoplasm of the vascular endothelial cells surrounding the MCD, while the level of TonEBP was much lower in the collecting ducts from the cortex or the medulla. In the interstitial cells, TonEBP was detected from F18 and increased steadily afterward. During renal development, the expression of NKCC2 is followed by that of TonEBP and its target genes AR, UT-A in DTL, and UT-A1 in IMCD. When the activity of NKCC2 is inhibited in neonatal animals, expression of TonEBP and its target genes is dramatically reduced. These data are consistent with the notion that hypertonicity generated by sodium reabsorption via NKCC2 drives expression and activation of TonEBP in the course of renal development. The expression of TonEBP and UT-A during development is likely a factor contributing to the urine concentrating ability of the mouse7).
In the hypokalemic animals, the abundance of TonEBP decreased significantly in the outer and inner medulla. Underlying mechanisms appeared different in the two regions because the abundance of TonEBP mRNA was lower in the outer medulla but unchanged in the inner medulla. Immunohistochemical examination of TonEBP revealed cell type-specific differences. TonEBP expression decreased dramatically in the outer and inner medullary collecting ducts, thick ascending limbs, and interstitial cells. In the descending and ascending thin limbs, TonEBP abundance decreased modestly. In the outer medulla, TonEBP shifted to the cytoplasm in the descending thin limbs. Transcription of aldose reductase, a target of TonEBP, was decreased since the abundance of mRNA and protein was reduced. Downregulation of TonEBP appeared to have also contributed to reduced expression of AQP2 and UT-A urea transporters in the renal medulla. In cultured cells expression and activity of TonEBP were not affected by reduced potassium concentrations in the medium. These data support the view that the medullary tonicity regulates expression and nuclear distribution of TonEBP in the renal medulla in cell type specific manners [unpublished data].
TonEBP has key roles in the protection of renal medullary cells from the deleterious effects of high concentrations of salt and urea that are produced as a result of the operation of the urinary concentrating mechanism and direct role in the urinary concentrating mechanism via stimulating urea permeability in the inner medullary collecting duct and possibly water permeability in the principal cells of the collecting duct. Recent studies suggest that TonEBP is a major regulator of urinary concentration. TonEBP stimulates the promoter of urea transporter UT-A1 and UT-A3 that provide urea permeability in the IMCD. In the kidneys of transgenic mice expressing an inhibitory form of TonEBP, expression of UT-A1 and UT-A2 is reduced8), indicating that UT-A2 in the DTL is also a target of TonEBP in addition to UT-A1. Mice deficient in either UT-A1/3 or UT-A2 display reduced urea accumulation in the renal medulla9, 10). Thus TonEBP appears to be a key regulator in the urea recycling that leads to the massive accumulation of urea in the papilla.
Acknowledgement
This work was supported by Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) to J.Y. Jung. (No. F01-2005-000-10301-0)
References
1. Sands JM. Molecular approaches to urea transporters. J Am Soc Nephrol. 2002; 13:2795–2806. PMID: 12397052.

2. Nakayama Y, Peng T, Sands JM, Bagnasco SM. The TonE/TonEBP pathway mediates tonicity-responsive regulation of UT-A urea transporter expression. J Biol Chem. 2000; 275:38275–38280. PMID: 10995747.

3. Cha JH, Woo SK, Han KH, Kim YH, Handler JS, Kim J, Kwon HM. Hydration status affects nuclear distribution of transcription factor tonicity responsive enhancer binding protein in rat kidney. J Am Soc Nephrol. 2001; 12:2221–2230. PMID: 11675398.

4. Kim YH, Kim DU, Han KH, Jung JY, Sands JM, Knepper MA, Madsen KM, Kim J. Expression of urea transporters in the developing rat kidney. Am J Physiol Renal Physiol. 2002; 282:F530–F540. PMID: 11832436.
5. Han KH, Woo SK, Kim WY, Park SH, Cha JH, Kim J, Kwon HM. Maturation of TonEBP expression in developing rat kidney. Am J Physiol Renal Physiol. 2004; 287:F878–F885. PMID: 15226152.

6. Lim SW, Ahn KO, Sheen MR, Jeon US, Kim J, Yang CW, Kwon HM. Downregulation of renal sodium transporters and tonicity-responsive enhancer binding protein by long-term treatment with cyclosporin A. J Am Soc Nephrol. 2007; 18:421–429. PMID: 17202415.

7. Lee HW, Kim WY, Song HK, Yang CW, Han KH, Kwon HM, Kim J. Sequential expression of NKCC2, TonEBP, aldose reductase, and urea transporter-A in developing mouse kidney. Am J Physiol Renal Physiol. 2007; 292:F269–F277. PMID: 16926446.

8. Lam AK, Ko BC, Tam S, Morris R, Yang JY, Chung SK, Chung SS. Osmotic response element-binding protein (OREBP) is an essential regulator of the urine concentrating mechanism. J Biol Chem. 2004; 279:48048–48054. PMID: 15347663.

9. Fenton RA, Chou CL, Stewart GS, Smith CP, Knepper MA. Urinary concentrating defect in mice with selective deletion of phloretin-sensitive urea transporters in the renal collecting duct. Proc Natl Acad Sci U S A. 2004; 101:7469–7474. PMID: 15123796.

10. Uchida S, Sohara E, Rai T, Ikawa M, Okabe M, Sasaki S. Impaired urea accumulation in the inner medulla of mice lacking the urea transporter UT-A2. Mol Cell Biol. 2005; 25:7357–7363. PMID: 16055743.

Fig. 1
Renal physiology of TonEBP. NKCC2 and NCC drive accumulation of salt in the medullary interstitium and the medullary ray of the cortex, respectively. The resulting hypertonicity stimulates TonEBP. Stimulation of SMIT, BGT1, AR, and TauT leads to cellular accumulation of organic osmolytes and protection from the deleterious effects of hypertonicity. Stimulation of UT-A1 and UT-A2 leads to accumulation of urea in the renal medulla, which provides the majority of osmotic gradient for urinaryconcentration. Although not directly demonstrated, stimulation of AQP2 would contribute further to urinary concentration by facilitating reabsorption of water. The high expression of HSP70 protects cells from the harmful effects of high urea in the renal medulla. [Figure obtained from Jeon US et al. Acta Physiol (Oxf) 187:241-247, 2006]
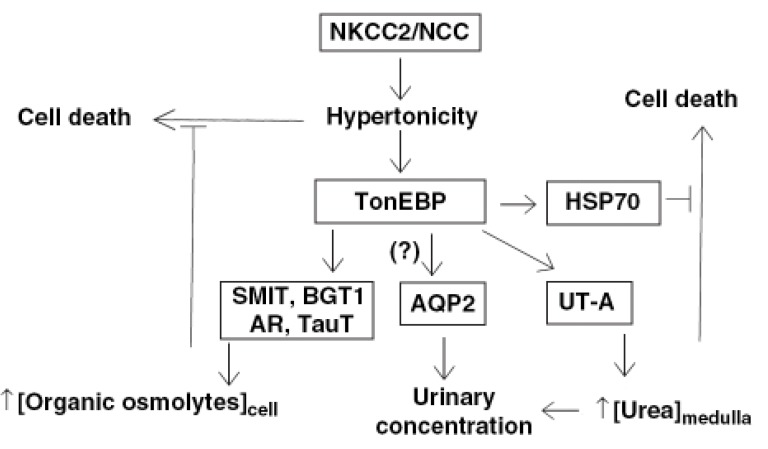
Fig. 2
Light micrographs of 50-µm thick Vibratome sections from kidneys of 7 (A)-, 14 (B)-, and 21-day-old (C) rat pups illustrating UT-A immunostaining. A: UT-A immunoreactivity was present only in the IMCD in the terminal half of renal papilla (arrowhead) and in the DTL of immature loops of Henle (open arrow) located at the border between outer medulla and inner medulla at 7 days after birth. B: UT-A immunoreactivity appeared in the terminal part of DTL of short loop of Henle (arrow) in the inner stripe of outer medulla at 14 days after birth. Note the strong immunoreactivity in the DTL of long loop of Henle (open arrow) and IMCD (arrowhead). C: in 21-day old pups, immunoreactivity for UT-A was increased in the DTL of short loop of Henle (arrow). Incontrast, the intensity of immunoreactivity in the long loop DTL (open arrow) was markedly decreased. [From Kim YH, Kim DU, Han KH, Jung JY et al.: Am J Physiol Renal Physiol 282:F530-F540, 2002]
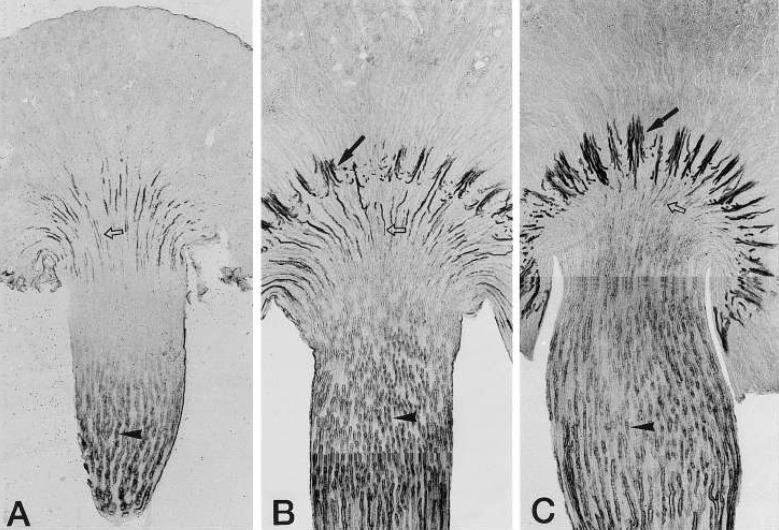
Fig. 3
Diagrams summarizing the expression profiles of NKCC2, TonEBP, AR, and UT-A in developing mouse and rat kidneys. Nuclear shift of TonEBP after birth is indicated. VE, vascular endothelial cells; CD, collecting duct cells; lDTL, long-looped descending thin limb; sDTL, shortlooped descending thin limb. [Diagram obtained from Lee HW et al. Am J Physiol Renal Physiol 292:F269-F277, 2007]




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download