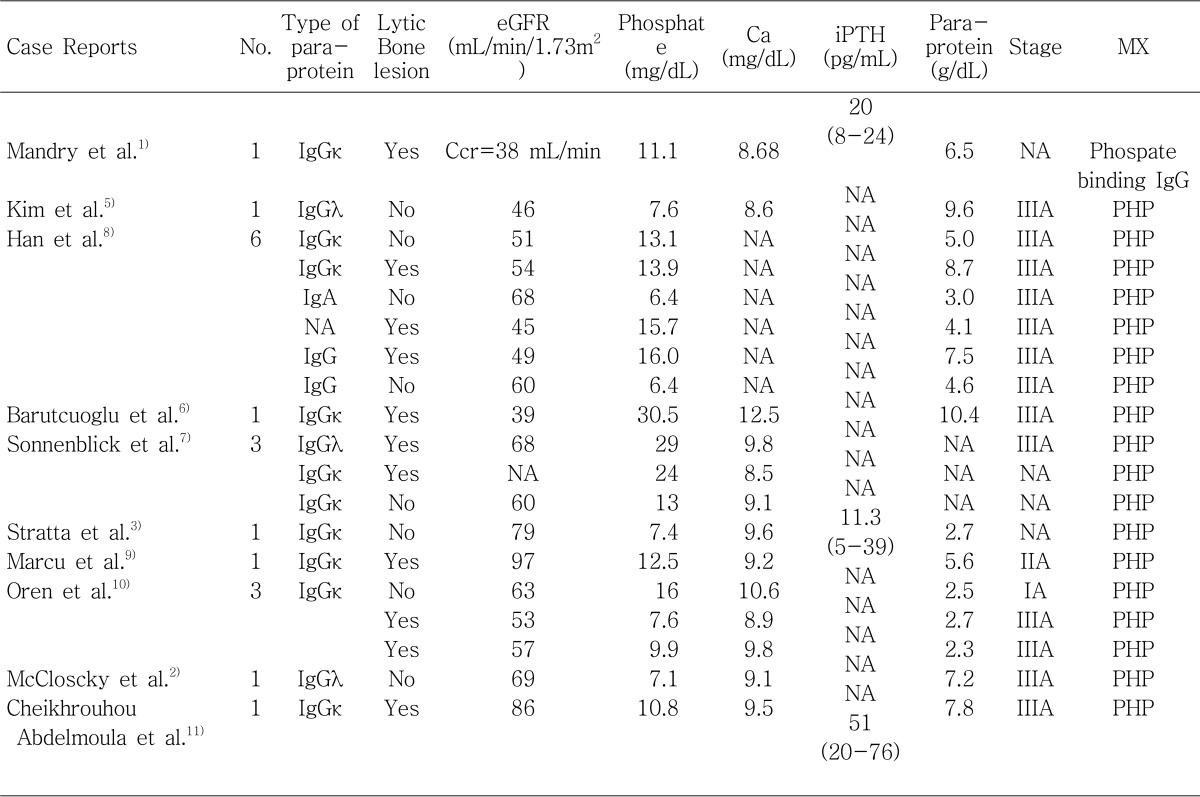
1. Mandry JM, Posner MR, Tucci JR, Eil C. Hyperphosphatemia in multiple myeloma due to a phosphate-binding immunoglobulin. Cancer. 1991; 68:1092–1094. PMID:
1913479.

2. McCloskey EV, Galloway J, Morgan MA, Kanis JA. Pseudohyperphosphataemia in multiple myeloma. BMJ. 1989; 299:1381–1382. PMID:
2513970.

3. Stratta P, Canavese C, Quaglia M, Lazzarich E, Morellini V, Brustia M, Bardone B, Bellomo G. A patient with unexplained hyperphosphataemia. Nephrol Dial Transplant. 2006; 21:2664–2666. PMID:
16766547.

4. Levey AS, Greene T, Kusek JW, Beck GJ. MDRD Study Group. A simplified equation to predict GFR from serum creatinine. [Abstract]. J Am Soc Nephrol. 2000; 11:155.
5. Kim KS, Kim DU, Lee WS, Chung WS. Pseudohyperphosphatemia in multiple myeloma. Korean J Hematol. 1994; 29:351–355.
6. Barutcuoglu B, Parildar Z, Mutaf I, Habif S, Bayindir O. Spuriously elevated inorganic phosphate level in a multiple myeloma patient. Clin Lab Haematol. 2003; 25:271–274. PMID:
12890170.
7. Sonnenblick M, Eylath U, Brisk R, Eldad C, Hershko C. Paraprotein interference with colorimetry of phosphate in Serum of some patients with multiple myeloma. Clin Chem. 1986; 32:1537–1539. PMID:
3731447.

8. Han JY, Lee JH, Choi BG, Moon YS, Jin SW, Han WH, Kim YG, Hong YS, Kim HK, Kim BK, Lee KS, Kim DJ. Clinical Spectrums of Pseudohyperphosphatemia in Multiple Myeloma. Korean J Med. 1996; 51:157–159.
9. Marcu CB, Hotchkiss M. Pseudohyperphosphatemia in a patient with multiple myeloma. Conn Med. 2004; 68:71–72. PMID:
15007869.
10. Oren S, Feldman A, Turkot S, Lugassy G. Hyperphosphatemia in multiple myeloma. Ann Hematol. 1994; 69:41–43. PMID:
8061106.

11. Cheikhrouhou Abdelmoula L, Amira C, Chaabouni L, Kchir MM, Zouari R. Hyperphosphatemia in multiple myeloma. Joint Bone Spine. 2003; 70:541–542. PMID:
14667570.

12. Adler SG, Laidlaw SA, Lubran MM, Kopple JD. Hyperglobulinemia may spuriously elevate measured serum inorganic phosphate levels. Am J Kidney Dis. 1988; 11:260–263. PMID:
3125742.

13. Teppo AM. Immunoturbidimetry of albumin and immunoglobulin G in urine. Clin Chem. 1982; 28:1359–1361. PMID:
6804127.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download