Discussion
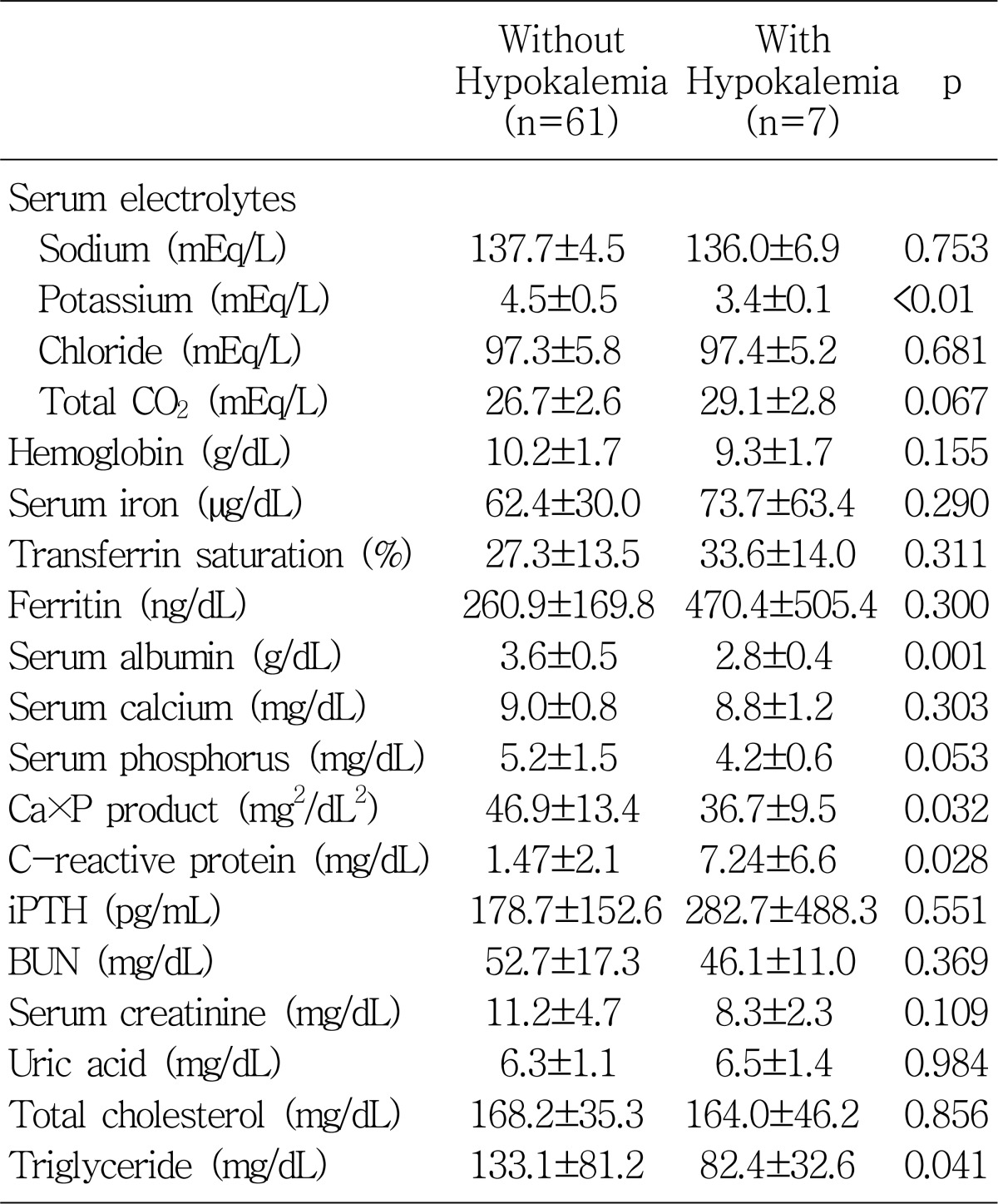
The occurrence of hypokalemia is strongly dependent on the patient population. In otherwise healthy adults not receiving any medication, less than 1% will develop hypokalemia
7). In CAPD patients, the prevalence of hypokalemia has been found to vary from the 10% to 36%
8). It was reported that 10-15% of PD patients required potassium supplementation for hypokalemia
9). In our study, the prevalence of hypokalemia in our center was about 10%, similar to previous studies
9).
In patients on chronic dialysis, hypokalemia is induced by factors affecting internal and external potassium balance. The internal potassium balance includes reduced potassium uptake by cells because of uremia, acidosis, drugs, and alterations of the profile of hormones influencing potassium movement and it represents the potassium redistribution between the intracellular and the extracellular compartments. Compared to hemodialysis, peritoneal dialysis patients have a normal or even higher intracellular potassium content, especially those on CAPD. This phenomenon is probably related to continuous glucose absorption from the dialysis solutions and the subsequent stimulation of intracellular uptake of potassium, mediated by insulin release
10). The external balance includes diminished or absent renal excretion, diminished dietary intake, and increased fractional excretion of potassium in the feces. The mode and frequency of dialysis also affected both internal and external potassium balance
11). Considered from a standpoint of internal potassium redistribution, the trend of a higher frequency of hypokalemia in diabetic patients in our study is explained by the hypothesis that the insulin hormone stimulated by hyperglycemia together with the continuous peritoneal glucose absorption, could promote an excess of potassium redistribution into the intracellular compartment by activating N
+ ,K
+-ATPase, which results in active potassium uptake
7). From the view of external balance, generally dialysis itself is the main source of external loss.
In CAPD, most dialysate solutions contain no potassium and patients dialyzed with such solutions lose 25-30 mEq of potassium per day via CAPD. Because this amount is relatively small when compared to the normal daily uptake (70-80 mEq)
9), hypokalemia secondary to low potassium ingestion usually occurs only after an extended period of low oral potassium intake. It was reported that serum potassium levels in PD patients were not only associated with nutritional status or severity of coexisting comorbid conditions, but were also an independent prognostic indicator in PD patients
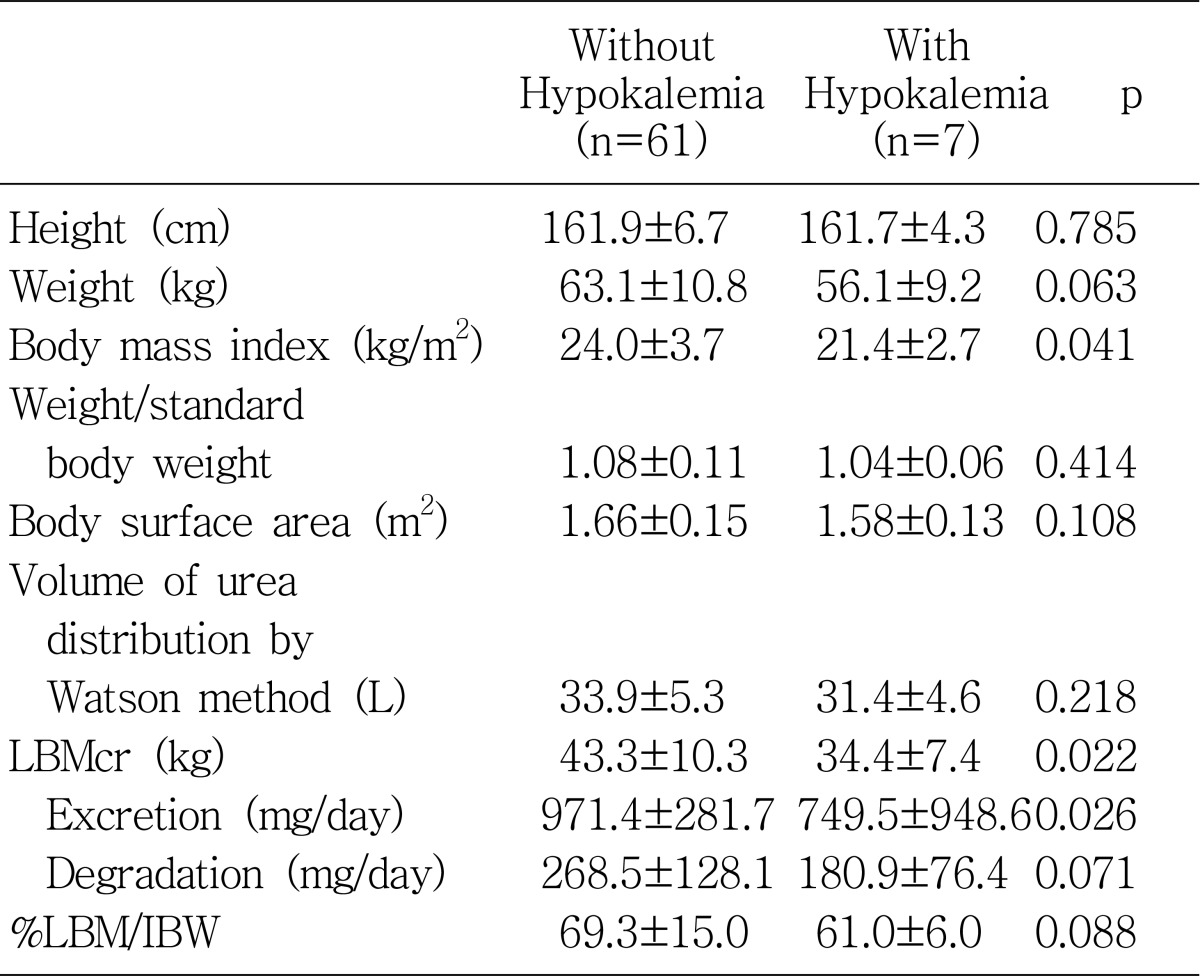
2). In accordance with this report, we also found that hypokalemia levels were associated with poor nutritional indicators such as lower PNA, body mass index, LBMcr, and serum albumin level.
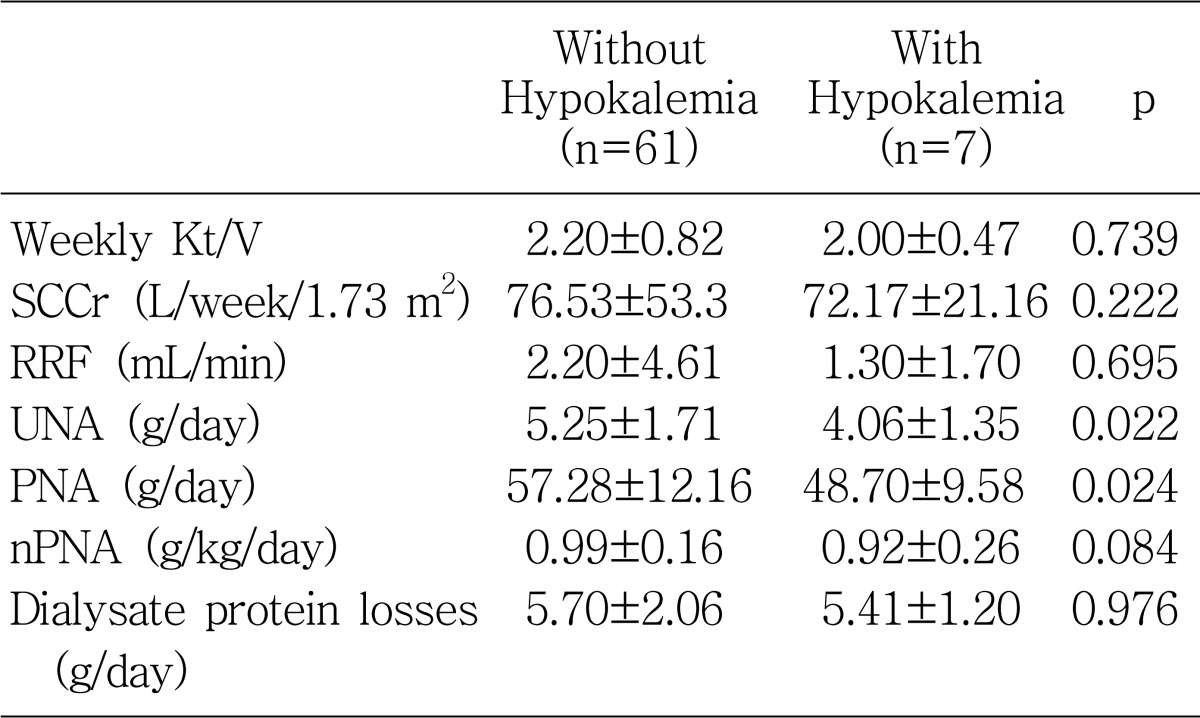
Protein equivalent of nitrogen appearance computed by urea kinetic modeling is known to reflect the dietary protein intake
5), which is closely related to dietary potassium intake since dietary protein contains about 1 mEq of potassium per gram
12). This study also revealed that dialysate urea nitrogen losses were significantly higher in the patients without hypokalemia. This can be probably explained by the nutritional aspect, because dialysate urea nitrogen loss is the main component of PNA. Some high potassium foods are also high in phosphorus; examples include corn, yogurt, milk, and beans
13). Although not reaching statistical significance, the serum potassium level was associated with the serum phosphate level and also significantly associated with calcium-phosphate, indirectly suggesting that hypokalemic patients had an overall reduction in general oral intake.
Serum CRP is an acute-phase protein that is a marker for underlying systemic inflammation. The prevalence of an increased CRP and other pro-inflammatory cytokines (interleukin-1, interleukin-6 and tumor necrosis factor-α) was reported to be high in dialysis and pre-dialysis patients
14). It is well documented that high levels of pro-inflammatory cytokines may cause muscle wasting by stimulating protein catabolism via the ubiquitin-proteosome pathway
15) and by inhibiting appetite
16); they are also associated with a higher cardiovascular mortality in ESRD patients
17). Qureshi et al. showed a close relationship between CRP and nutritional status as assessed by SGA (subjective global assessment)
18). Noh et al. reported that serum CRP is an independent predictor of two-year patient survival in CAPD patients
19). Recently, it was reported that malnutrition-inflammation scores including serum albumin level, total iron binding capacity level, BMI, etc. correlated significantly with clinical, nutritional, inflammatory, and anthropometric parameters in PD patients, even more strongly than SGA
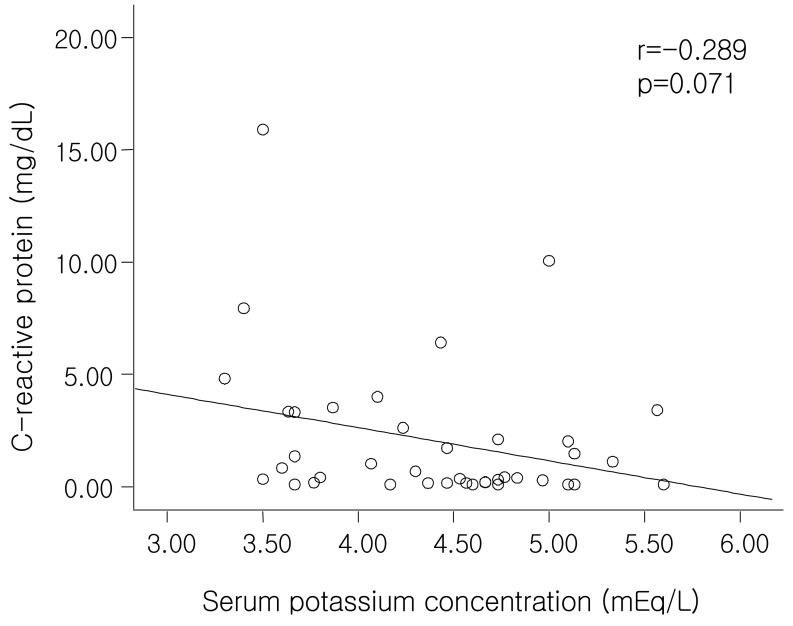
20). In our study, we also found that the patients with hypokalemia had a significantly higher CRP level; that level was negatively related to the serum albumin level (r=-0.29, p=0.07) (
Fig. 3) and serum potassium level (r=-0.289, p=0.071) (
Fig. 5), but these two correlations were not statistically significant. In addition, the higher levels of ferritin in the patients with hypokalemia in our study might also be explained in terms of an inflammatory marker.
The creatinine kinetic approach to lean body mass (LBM) estimation was reported to correlate well with other techniques for lean body mass, such as bioimpedance, infrared, and anthropometric measurements
4). We found that the patients with hypokalemia had significantly lower LBM levels than those without hypokalemia and that serum potassium levels correlated significantly with LBM levels (r=0.31, p=0.01). The total amount of potassium in the plasma did correlate with total body potassium in normal subjects
21), and was expected to bear close relationship to plasma potassium especially when maintained for sufficient time at steady levels
22). A decrease in total body potassium could either reflect a diminishing cellular mass, which is likely to be present in malnutrition, or a change in membrane function that is common in uremia
23).
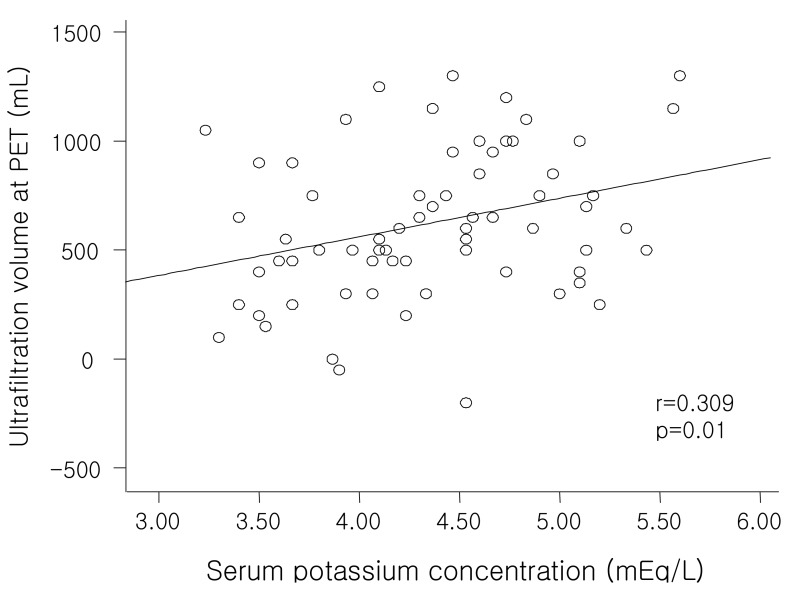
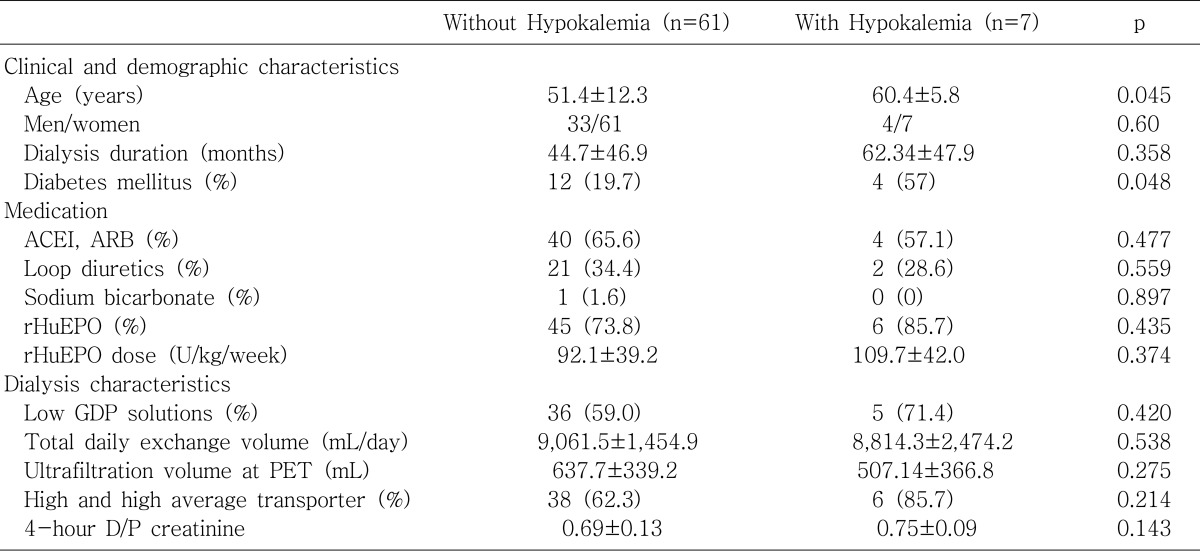
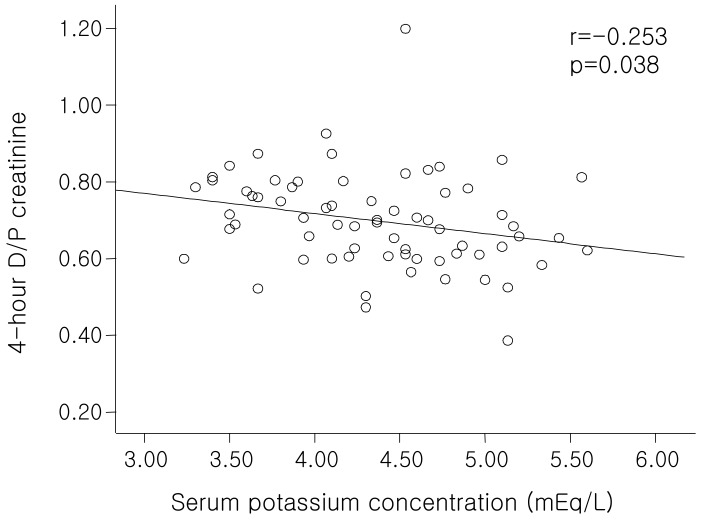
The ultrafiltration volume at the PET was significantly lower in patients with hypokalemia than those without hypokalemia (
Table 2) and showed a positive correlation with serum potassium levels both significantly and independently (
Fig. 2;
Table 6), consistent with the significant negative correlation between serum potassium level and 4-hour D/P creatinine (
Fig. 4). This is explained by both the higher rate of diffusive removal of potassium through the peritoneal membrane as a solute with a low molecular weight and the volume overload induced by the lower rate of fluid removal resulting from early loss of osmolar gradients. Cheng et al. also showed that there was a strong association between fluid status and nutritional status, and that volume overload is associated with the development of malnutrition with inflammation. Furthermore, improved fluid status was associated with improvement in nutritional status
24). This can be interpreted by the hypothesis that volume overload might lead to gastrointestinal edema with poor nutritional status which results in bacterial and endotoxin translocation. This phenomena can elevate serum TNF-α and other inflammatory cytokines which induce anorexia by inhibiting the normal feeding response to energy deficits
25). We also showed in our study that lower ultrafiltration volume at a PET with higher 4-hour D/P creatinine is associated with hypokalemia, probably reflecting volume overload with poor nutritional status. We did not find any correlation between serum potassium level and daily exchange volume or the dialysis adequacy index Kt/V, as with a previous study done by Szeto et al.
2).
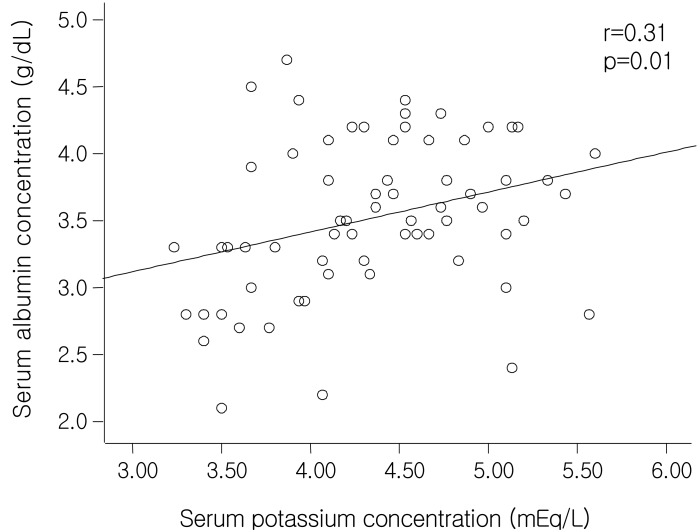
It has been well established that serum albumin level is a strong predictor of increased risk of morbidity and mortality in peritoneal dialysis patients
26). The amount of albumin in the body and serum albumin concentration are determined by multiple factors including hepatic albumin synthesis (which is influenced by dietary protein intake), protein catabolism, and extravascular albumin distribution
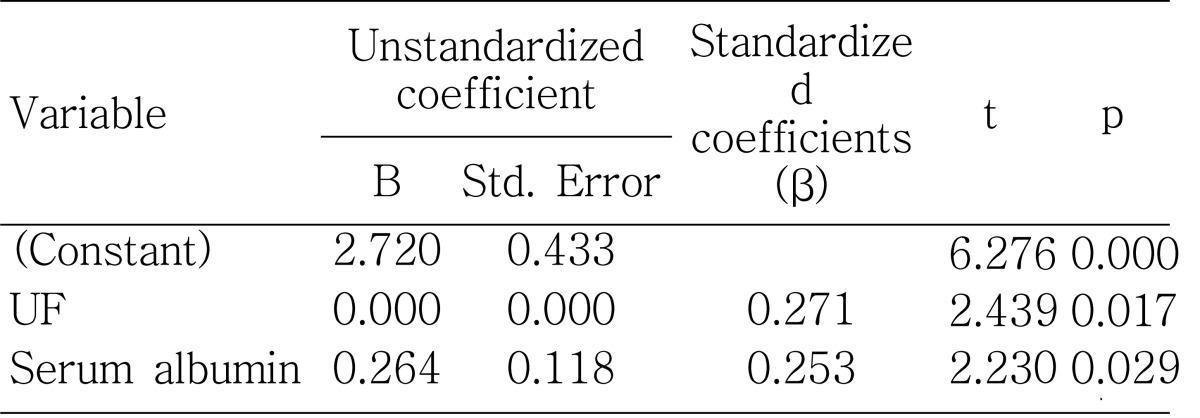
27). In addition to these factors, CAPD patients are uniquely susceptible to the adverse effects on levels of serum albumin because of protein losses into the peritoneal effluent. Our study showed that the patients with hypokalemia had significantly lower serum albumin levels than those without hypokalemia and that the serum albumin levels correlated significantly with serum potassium levels (r=0.31, p=0.01), even after adjustment by 4-hour D/P creatinine, total body water by the Watson method and dialysate albumin losses by partial correlation analysis (r=0.255, p=0.041). This suggests that none of the factors-potassium loss via the peritoneum as a small molecule or hypoalbuminemia caused by overhydration or excessive peritoneal loss of albumin can explain the relationship between serum albumin and potassium levels sufficiently. Stepwise multiple linear regression analysis also showed that serum albumin levels reveal independently-significant correlation with serum potassium levels (β=0.253, p=0.029), together with ultrafiltration volume at the PET (β=0.271, p=0.017) (
Table 6). Higher levels of triglyceride, blood urea nitrogen, and serum creatinine in the patients without hypokalemia can also be explained by the common association with nutritional status.
In summary, we have shown that the patients with hypokalemia were associated with older age, higher CRP levels, and the presence of diabetes mellitus. Serum albumin, calcium-phosphate product, triglyceride, BMI, PNA, and LBMcr were significantly lower as compared to those without hypokalemia. The serum potassium level revealed significant positive correlation independently with ultrafiltration volume at the PET and serum albumin level. These results emphasize the importance of serum potassium level as a valid nutritional marker as well as a potential predictor of outcome. Further long-term follow up of a larger number of patients would be needed to clarify this relationship.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download