Abstract
A 52-year-old woman was referred to our hospital due to chronic renal failure with a 10-year history of hypertension. We found polycystic kidney disease, pulmonary tuberculosis and an aldosterone-producing adrenocortical mass. At this time, her serum potassium level and blood pressure were within the normal range. She refused hemodialysis and then was hospitalized because of uremic encephalopathy. On admission, her serum potassium level was normal without treatment and plasma aldosterone concentration highly elevated. She received hemodialysis, and thereafter hypokalemia developed. We then administered spironolactone, whereupon serum potassium level returned to the normal range. In this case, we thought that normokalemia was balanced hypokalemia of primary aldosteronism with hyperkalemia of chronic renal failure, and that hypokalemia developed after hemodialysis was due to an imbalanced primary aldosteronism with end stage renal disease.
Primary aldosteronism, which was first reported by Conn in 19551), is characterized by hypertension, hypokalemia, increased plasma aldosterone concentration (PAC) and decreased plasma renin activity (PRA). Since then, various clinical features of primary aldosteronism have been reported2). In case of primary aldosteronism with chronic renal failure, there is difficult to be diagnosed because hypertension is frequently associated with chronic renal failure. A few cases with primary aldosteronism accompanying chronic renal failure or end stage renal disease have been reported3-5). Two cases of primary aldosteronism with chronic renal failure6, 7) and one case with autosomal dominant polycystic kidney disease8) have been reported in Korea. All of cases had typical clinical manifestations of primary aldosteronism which of hypertension, hypokalemia, increased plasma aldosterone concentration and decreased plasma renin activity. Then, we report primary aldosteronism which has atypical clinical manifestation because of end stage renal disease.
A 52-year-old woman was referred to our hospital for evaluation of renal insufficiency. She complained of general weakness, anorexia, and weight loss. She had been treated with antihypertensive drugs for 10 years. Her brother had polycystic kidney disease. Her blood pressure was 110/80 mmHg and other physical findings were unremarkable. Laboratory findings were hemoglobin 5.9 g/dL, white blood cell count 13,350/mm3 with left shifting, platelet count 497,000/mm3, serum sodium 136 mEq/L, potassium 3.2 mEq/L, chloride 106 mEq/L, calcium 8.0 mg/dl, phosphorus 5.4 mg/dL, magnesium 2.5 mg/dL and spot urine potassium 14.3 mEq/L. The blood urea nitrogen level was 94 mg/dL, creatinine 7.1 mg/dL and creatinine clearance 7 ml/min. Blood gas analysis showed pH 7.356, PCO2 31.2 mmHg, PO2 108.5 mmHg, HCO3- 18.8 mmol/L, consistent with metabolic acidosis. The electrocardiogrphy showed a normal sinus rhythm. The chest X-ray revealed an infiltration in the right upper lobe. The renal sonography revealed multiple cysts in both kidneys, and the right kidney was measured 11.3 cm, left kidney 11.2 cm.
We thought that chronic renal failure was due to autosomal dominant polycystic kidney disease and that hypokalemia was due to diuretics. Thus, we ceased administration of diuretics, and we also ceased administration of antihypertensive drugs because blood pressure was normotensive. Pulmonary tuberculosis was treated and arteriovenous fistula was created in preparation for hemodialysis. We examined the hormones because hypokalemia remained the same in spite of the cessation of administration of diuretics. She was then discharged.
In the office, the blood pressure was 120/80 mmHg and serum potassium 4.3 mEq/L. Plasma renin activity decreased to 0.18 ng/ml/h (normal : 1.3-3.95 ng/mL/h) and plasma aldosterone concentration increased to 99.3 ng/dl (normal : 4.0-31 ng/dL). The plasma aldosterone concentration to plasma renin activity ratio was 551.6 ng/dL per ng/mL/h. The serum levels of ACTH, cortisol and thyroid hormones were within the normal range.
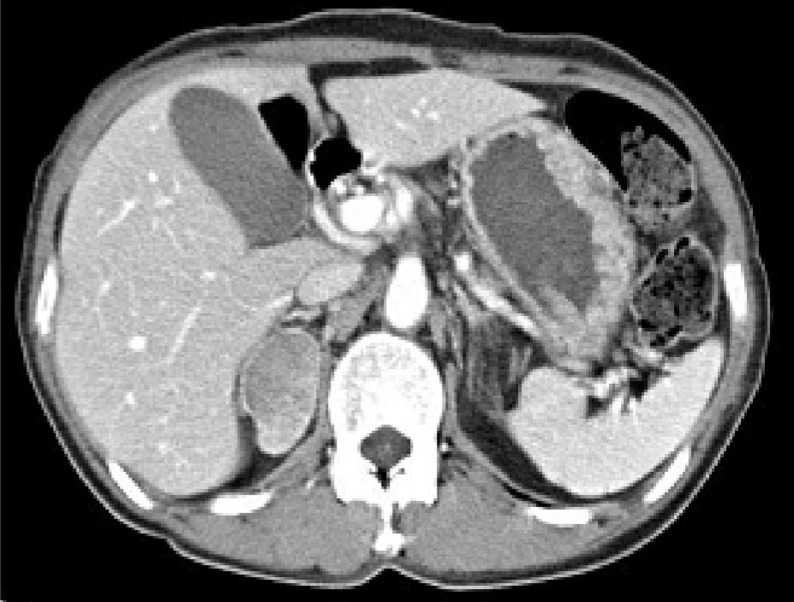
Abdominopelvic computed tomography revealed a 4.4 cm×2.8 cm-sized mass lesion in the right adrenal gland (Fig. 1). We surmised that this was aldosterone-producing adenoma in end stage renal disease. So, we recommended hemodialysis and operation for the adrenal mass. She refused hemodialysis on the ground of religion.
Two months later, she revisited our hospital because of uremic encephalopathy. Her blood pressure was 100/80 mmHg. Her blood urea nitrogen level was 173 mg/dL and serum creatinine level was 16.7 mg/dl. The serum potassium level was 4.8 mEq/L and spot urine potassium level was 26.4 mEq/L. The plasma renin activity was 3.3 ng/mL/h (normal : 1.3-3.95 ng/mL/h) and plasma aldosterone concentration had increased greatly to 2161 ng/dL (normal : 4.0-31 ng/dL). She received emergent hemodialysis and her uremic symptoms were improved.
After 5 days, she complained of both leg weakness and numbness. At this time, her serum potassium level was 2.9 mEq/L and spot urine potassium level was 16.1 mEq/L. We prescribed spironolactone 100 mg, and her blood pressure and serum potassium level were normal. She is now receiving maintenance hemodialysis. We plan to operate on her right adrenal gland mass if her general condition improves.
Primary aldosteronism is usually associated with hypokalemia, hypertension and metabolic alkalosis1). Hypokalemia is one of the most important symptoms in primary aldosteronism, and the mechanism of this hypokalemia is recognized to be mainly caused by accelerated potassium excretion in renal distal tubules responding to excess aldosterone. The arterial hypertension of primary hyperaldostrenosim is usually explained by an increase in sodium reabsorption related to the effect of aldosterone on distal renal tubules. This transient hypervolemia, along with the direct effects of aldosterone, induces secretion of natriuretic factors such as atrial natriuretic peptide (ANP)9). Blood pressure remains high because of several factors, including increased peripheral vascular resistance, a direct hypertensive effect of aldosterone on the central nervous system, and increased vascular sensitivity to pressor substances such as angiotensin and adrenalin. However, the clinical and biological spectrum of primary aldosteronism varies9). In particular, hypokalemia is lacking in 7 to 38% cases2). Normotensive primary aldosteronism is very rare10) and is not fully understood. In particular, primary aldosteronism with chronic renal failure is difficult to be diagnosed because chronic renal failure is usually associated with hypertension and two cases have been reported in Korea6, 7).
The aldosterone accelerated renal excretion of potassium, whereas augmented potassium metabolism by aldosterone is also observed partly in the gastrointestinal tract, salivary glands, and sweat glands. Therefore, in primary aldosteronism with chronic renal failure, the serum potassium level seems to be determined by the balance of the augmentation and the suppression of potassium excretion by excess aldosterone and renal failure, respectively3, 5).
In this case, we thought that normokalemia was balanced hypokalemia of primary aldosteronism with hyperkalemia of chronic renal failure, and that hypokalemia developed after hemodialysis was due to an imbalanced primary aldosteronism with end stage renal disease. In a word, the effect of excess aldosterone and the grade of renal failure should be almost counter-balanced. Plasma renin activity was not suppressed in the follow-up test and we thought that plasma renin activity escaped from the suppression by the automonously secreted aldosterone or stimulated by chronic renal failure11, 12). We conclude that when a patient with chronic renal failure shows unexplained hypokalemia, an association with primary aldosteronism should be considered. And normal blood pressure and plasma renin activity do not exclude the diagnosis of primary aldosteronism with chronic renal failure. Especially, in case of primary aldosteronism with hemodialysis, serum potassium level have to be monitored frequently.
References
1. Conn JW. Primary aldosteronim: A new clinical syndrome. J Lab Clin Med. 1955; 45:6–17.
2. Bravo E, Tarazi R, Dustan H, Fouad F, Textor S, Gifford R, et al. The changing clinical spectrum of primary hyperaldosteronism. Am J Med. 1983; 74:641–651. PMID: 6340491.
3. Matsuda K, Shimamoto K, Ura N, Ogata H, Shizukuda Y, Iwakura M, Nozawa A, Kikuchi K, Iimura O. A case of primary aldosteronism with chronic renal failure undergoing hemodialysis treatment. Endocrinol Jpn. 1989; 36:681–686. PMID: 2620664.

4. Nakada T, Kimura M. Primary aldosteronism associated with chronic renal failure : Report of a case. Int Urol Ntphrol. 1983; 16:165–173.
5. Koshiyama H, Fujisawa T, Kuwamura N, Nakamura Y, Kanamori H, Oida E, Hara A, Suzuki T, Sasano H. A case of normoreninemic aldosterone-producing adenoma associated with chronic renal failure. Endocrine. 2003; 21:221–226. PMID: 14515005.
6. Sung JR, Song KI, Kim JH, Cha MK, Lee EY, Park MS, Han DC, Jin SY, Hwang SD, Moon C, Lee HB. A case of primary aldosteronism, nephrotic syndrome and chronic renal failure: a diagnostic dilemma. Korean J Nephrol. 1997; 16:162–166.
7. Kim YS, Choi HY, Kim HJ, Kim DK, Jung IH, Lee TH, Yoon SY, Choi KH, Kang SW, Lee HY, Han DS, Chung HJ. Primary aldosteronism complicated with chronic renal failure. Korean J Nephrol. 2003; 22:156–160.
8. Park DJ, Yang JI, Choi YM, Kang MJ, Kim HJ, Lee SS, Lee JD, Chang SH, Chung SI, Kwon SI. Autosomal dominant polycystic kidney disease complicated by aldosterone-producing adrenal adenoma. Korean J Nephrol. 1998; 17:968–972.
9. Zimmerman RS, Edwards BS, Schwab TR, Heublein DM, Burnet JC. Atrial natriuretic peptide during mineralocorticoid escape in the human. J Clin Endocrinol Metab. 1987; 64:624–627. PMID: 2950127.

10. Vantyghem MC, Ronci N, Provost F, Ghulam A, Lefebvre J, Jeunemaitre X, Tabarin A. Aldosterone-producing adenoma without hypertension : a report of two cases. Eur J Endocrinol. 1999; 141:279–285. PMID: 10474126.
11. Saruta IT, Kondo K, Ozawa Y, Hata J, Shimizu K. Renin and juxtaglomerular apparatus in a patient with primary aldosteronism complicated by chronic renal failure. South Med J. 1976; 69:951–952. PMID: 941067.
12. Oelkers W, Diederich S, Bähr V. Primary hyperaldosteronism without suppressed renin due to secondary hypertensive kidney damage. J Clin Endocrinol Metab. 2000; 85:3266–3270. PMID: 10999820.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download