Abstract
The kidney is an important organ for controlling the volume of body fluids, electrolytic balance and excretion/reabsorption of endogenous and exogenous compounds. Among these renal functions, excretion/reabsorption of endogenous and exogenous substance is very important for the maintenance of physiological homeostasis in the body. Recently discovered organic anion transporters (OAT or SLC22A) have important roles for renal functions. It is well known as drug transporter. Several isoforms belong to SLC22A family. They showed different transport substrate spectrums and different localizations within the kidney. Their gene expressions are changed by some stimulus. The functional transport properties are regulated by protein kinase C. In addition, the function of organic anion transporters are also regulated by protein-protein interaction, such as caveolin which is compositional protein of caveolae structure. In this review, we will give an introduction of organic anion transporters and its regulatory mechanisms.
The kidney, as well as the liver, plays a primary role in the excretion of endogenous/exogenous compounds and their metabolites1-5). In addition to glomerular filtration, the kidney excretes charged compounds via carrier-mediated pathways, which are organic anion transport pathways, in renal proximal tubular cells6-9). Particularly the organic anion transport pathway has been shown to mediate the elimination of various drugs including anti-human immunodeficiency virus therapeutics, non-steroidal anti-inflammatory drugs, antitumor drugs, antibiotics, diuretics, and antihypertensives10). These compounds are chemically heterogeneous substances possessing a carbon backbone and a net negative or positive charge at physiological pH. A great variety of endogenous and exogenous substances that are harmful to the body can be classified into organic anions, and their elimination is therefore essential for the maintenance of homeostasis. Excretory organs such as the kidney, liver and intestine defend the body against potentially harmful effects of these compounds by biotransformation into less active metabolites and the excretory transport process. Particularly in the kidney, drugs and environmental toxicants are eventually excreted into the urine, either in the unchanged form or as biotransformation products. This renal excretion is closely related to physiological events occurring in nephrons, i.e., filtration, secretion, and reabsorption.
More than 80 years ago, it was already observed that phenolsulfonphthalein, an organic anion, was highly concentrated in the renal convoluted tubules, indicative of the tubular secretion process11). Transepithelial transport of organic anions in proximal tubules is carried out by two distinct transporters; first, organic anions are transported from the blood by basolateral organic anion transporter(s), and subsequently effluxed into the tubular lumen by luminal transporter (s). Until early 1990s, renal organic anion transport has been considered to be carried out by several carrier proteins that show wide substrate specificity. Various anionic compounds have been indicated to be taken up into the proximal tubular cells by the "classical PAH (p-aminohippurate) transporter". Recently, several organic anion transporters were reported from the kidney, liver, and olfactory mucosa10, 12, 13). In addition, there are carrier proteins to transport organic anions such as organic anion transporting protein, multi drug resistance protein or p-glycoprotein, and so on. Therefore, in order to help reader's comprehension, this review will focus on the present knowledge of molecular properties of renal organic anion transporters (OAT, SLC22A).
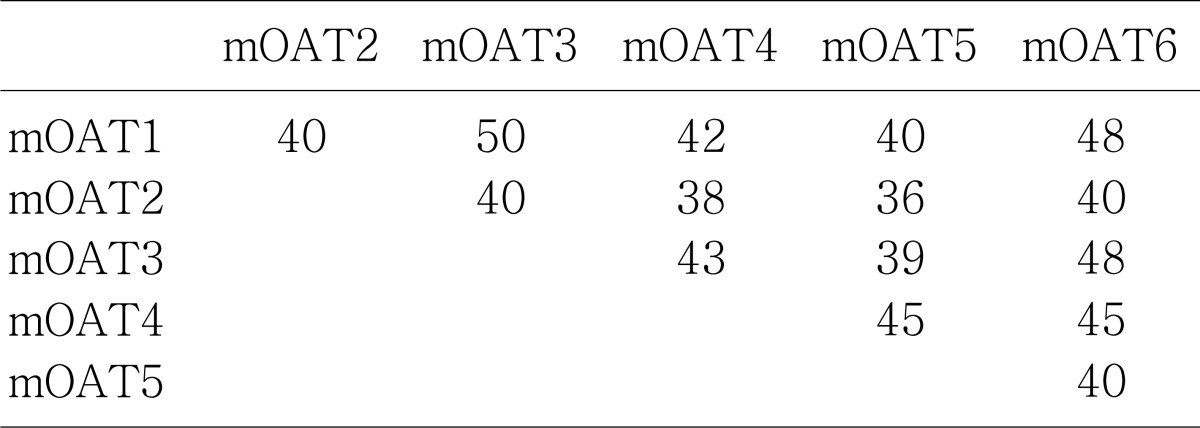
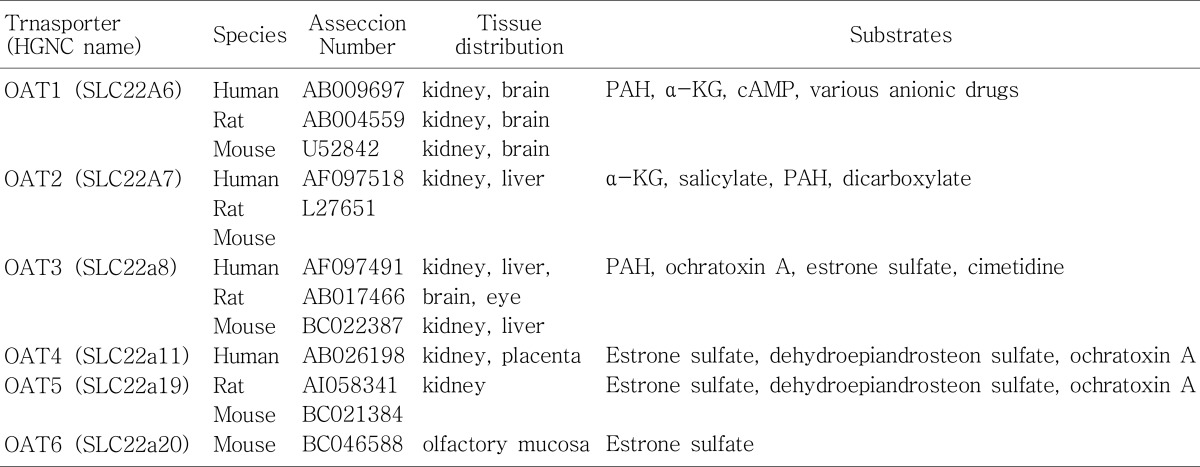
In 1997, Sekine and Sweet et al. independently isolated the PAH transporter protein from rat kidney (OAT1/ROAT1) employing the expressional cloning technique10, 14). OAT1 and ROAT1 are identical and will be collectively referred to as OAT1. OAT1 is a 551-amino-acid protein with 12 putative transmembrane domains (Fig. 1). Other members of OAT have 535-568 amino acid residues and also have 12 membrane spanning domains. The molecular structure of OAT1 has no similarity with those of other members of polyspecific organic anion transporter families, e.g., the organic anion transporting polypeptide (oatp) or the multidrug resistance-associated protein (Mrp) families. The amino acid sequence alignment of OATs shows that they have relatively high homology from 36% to 50% (Table 1).
The all members of the OAT family have several protein kinase C (PKC) sites in predicted large loops between the sixth and seventh membrane spanning domains (Fig. 1). The transport function of OAT1, OAT3 and OAT4 is regulated by PKC. The PAH transport by rOAT115) and hOAT116) expressed in Xenopus laevis oocytes and in HeLa cells, respectively, was inhibited after treatment of cells with phorbol esters. This phorbol-ester-induced inhibition of PAH transport was prevented by staurosporin, suggesting an inhibitory role of protein kinase C in OAT1. The uptake functions of [3H] estrone sulfate or [3H] dehydroepiandrosterone sulfate by OAT3 and OAT4 in overexpressed S2 cells or BeWo cells were also attenuated by the existence of phorbol-ester, a protein kinase C activator17, 18). In addition, the putative phosphorylation sites for protein kinase A, casein knase II, and tyrosine kinase have also been reported. Whether any of these sites is used for the regulation of OATs is as yet unclarified.
All OATs have several N-glycosylation sites between the first and second transmembrane domains. Inhibition of glycosylation by tunicamycin in mouse OAT1-expressing COS-7 cells resulted in an increase of intracellular accumulation of newly synthesized transporters, suggesting that glycosylation is required for insertion of OAT1 into the plasma membrane19).
A number of endogenous and exogenous organic anions are secreted in the renal proximal tubules. The active transepithelial secretion of organic anions into the urine is mediated by two types of transporter proteins existing in the basolateral and luminal membranes of the renal proximal tubules. The first step is the uptake. Tubular secretion of organic anions occurs in two transmembrane transport processes (Fig. 2). When considering the electrical potential inside a cell of the proximal tubule, the basolateral uptake of organic anions is an uphill transport process. In 1987, Shimada and coworkers reported that uptake of paraaminohippuric acid (PAH), a typical substrate of renal organic anion transport system, was dramatically enhanced in the presence of an outward α-ketoglutarate gradient20). The PAH is taken up by proximal tubule cells in exchange for intracellular dicarboxylates, that are then returned to the cells via the Na+-dicarboxylate cotransporter. These findings indicate that the PAH transporter is a tertiary active transporter. Na+, K+ ATPase (a primary active transporter) generates the sodium ion gradient between the plasma membrane21), and the electrochemical gradient of the sodium ions derives the transport of dicarboxylates via the Na+-dicarboxylate cotransporter (a secondary active transporter)22). Using the gradient of dicarboxylates, the PAH transporter takes up organic anions in exchange for the dicarboxylates (a tertiary active transporter). In addition to this classical PAH transporter, an additional uptake system has been characterized employing bulky organic anion fluoresceinmethotrexate as a substrate23). Uptake of fluoresceinmethotrexate is independent of Na+ and is not inhibited by PAH or glutarate. The molecular identity of this transporter has not yet been established.
The transport properties of OAT1 has been characterized functionally using Xenopus oocytes and the cell expression system (Table 2). The cloned OAT1 has been expressed in Xenopus laevis oocytes10, 14-16, 24-26), COS-7 cells19), or HeLa cells16). The uptake ratio of radiolabeled PAH in cells expressing these carriers was increased compared with that water-injected oocytes or mock-transfected cells, respectively. The reported Km values range between 5 and 70 µM (5 and 9 µM for hOAT116, 25); 37 µM for mOAT119); 14 and 70 µM for rOAT110, 14) and 21 µM for flounder OAT127)). Uptake of PAH by rOAT1, hOAT1, and fOAT1 was trans-stimulated by intracellulary accumulated α-ketoglutarate or glutarate. Similarly, efflux of radiolabeled glutarate from OAT1-expressing oocytes was trans-stimulated by PAH in the incubation medium28). It was proved that OAT1 acts as a PAH/dicarboxylate antiporter. In addition to PAH, rOAT1 mediated the transport of various organic anions, including endogenous organic anions, such as prostaglandin E2 (PGE2), cyclic guanosine monophosphate (cGMP), cyclic adenosine monophosphate (cAMP), dicarboxylic acid and urate, and exogenous ones, including a number of anionic drugs such as β-lactams, nonsteroidal antiinflammatory drugs (NSAIDs), antiviral drugs, and methotrexate10, 15, 28-31). The affinities of these compounds for OAT1 were very similar to those reported for those of the basolateral PAH transporter. In addition, the hydrophobicity and the strength of the anionic charge of substrates appear to be closely related to their affinity for OAT132). OAT1 also transports ochratoxin A33) that is well known as the etiologic agent responsible for Balkan nephropathy. When OAT1-overexpressing cells and mock-transfected cells were exposed to ochratoxin A, decreased cell viability was observed in OAT1-expressing cells, which were recovered by inhibition of the OAT1-mediated uptake of ochratoxin A. This result suggests that OAT1 is associated with proximal tubular damage caused by anionic toxicants33, 34).
Recently, an electrophysiological approach was used to demonstrate translocation of organic anions by fOAT1-expressing Xenopus oocytes35). PAH, etacrynic acid, bumetanide, and tienilic acid evoked an inward current. In contrast to these compounds, probenecid and furosemide did not generate an inward current.
The tissue distribution of cloned rat, mouse, and human OAT1 is investigated by northern blot analysis (Table 2)10, 14-16, 19, 25, 26). rOAT1, mOAT1, and hOAT1 mRNAs are expressed predominantly in the kidney and very weakly in the brain. In the rat kidney, rOAT1 is localized in the middle portion of proximal tubules (S2 segment) as determined by in situ hybridization10). Immunohistochemical localization also demonstrated the rat36, 37) and human OAT125) proteins are restricted to the S2 segment of proximal tubular cells. Western blot analysis revealed that the rOAT1-encoded protein size was about 77 kDa. This rOAT1 protein was up-regulated by infusion of chronic furoemide or hydrochlorothiazide38).
OAT2 has cloned by the membrane protein previously called the novel liver transporter (NLT), but whose transport substrates had not been identified39). Rat OAT2 had 42% amino acid sequence homology with rOAT1, and was shown to be a member of the OAT family (Table 1)40). rOAT2 mediated the sodium-independent transport of salicylate (Km=88 µM) and α-ketoglutarate (Km=18 µM). rOAT2 also mediated the transport of PGE2, methotrexate, acetylsalicylate, and PAH (Table 2). rOAT2 is a 535-amino-acid protein with 12 putative membrane-spanning domains. rOAT2 mRNA is predominantly expressed in the liver, and weakly in the kidney. Immunohistochemical localization also demonstrated the rat OAT2 protein is detected in the apical surface of the tubule in the medullary thick ascending limb of Henle's loop and cortical and medullary collecting ducts. Western blot analysis revealed that the rOAT2-encoded protein size was about 60 kDa41). Mouse OAT2 was isolated and characterized in 200242). Different from rOAT2, mOAT2 is 540-amino-acid protein having 88% homology with rOAT2. Gene expression of mOAT2 was quite different from that of rOAT2. In contrast to rOAT2 mRNA was expressed predominantly in liver, mOAT2 mRNA was not detected in liver with Northern blot analysis. This mOAT2 mRNA was weakly detected in female liver but not kidney. In substrate specificity, mOAT2 mediated the sodium-independent transport of glutarate (Km=15.8 µM) and PGE2 (Km=5.2 µM). Interestingly, no transport of salicylate, prototype substrate of rOAT2, was detected. The gene expression of rOAT2 in isolated hepatocytes were down-regulated by the treatment with nitric oxide43).
Rat OAT3, another member of the multispecific organic anion transporter family, was isolated from the rat brain by the RT-PCR cloning method based on the sequence conserved among rOAT1, rOAT2, rOAT5 and mOAT644).
rOAT3 shows 49, 39, 37 and 48% identity with rOAT1, rOAT2, rOAT5 and mOAT6, respectively (Table 1). When expressed in Xenopus oocytes, rOAT3 mediated the uptake of organic anions such as estrone sulfate (Km=2.3 µM), ochratoxin A (Km=0.74 µM), and PAH (Km=65 µM), and cimetidine (Table 2). OAT3 also interacted with anionic neurotransmitter metabolites, such as epinephrine, norepinephrine, and serotonin. The transport mode of OAT3 was sodium-independent, and no trans-stimulatory effect of estrone sulfate, PAH, or ochratoxin A was detected in used Xenopus oocyte expression system. When rOAT3 is expressed in cells, rOAT3-mediated estrone sulfate uptake is enhanced compared with that in the case of mock-transfected cells. When cRNA injected Xenopus oocyte or renal slice were preloaed with dicarboxylate, the transport of substrate mediated rOAT3 was enhanced. This fact shows that rOAT3 is also exchanger protein45). In 1999, Race et al. reported hOAT326). They failed to detect any functional properties of hOAT3. Cha et al. also reported hOAT3 in 200146). This newly cloned hOAT3 transported estrone sulfate (Km=3.2 µM), PAH (Km=87.2 µM), methotrexate (Km=10.9 µM), cimetidine (Km=57.4 µM), dehydroepiandrosterone sulfate, estradiol glucuronide, glutarate, PGE2 and so on. The difference of these two clone was that the amino acid sequences of C-terminus of newly identified hOAT3 was shorter than those of old hOAT3. rOAT3 mRNA was expressed in the liver, kidney and brain, and a weak expression was detected in the eye. hOAT3 mRNA was detected mainly in the kidney and very weakly in the brain and smooth muscle. The distribution of hOAT3 protein was observed at the basolateral membrane of proximal tubule cells
Recently, we identified the fourth member of organic anion transporter expressed in the human placenta using EST (expressed sequence tag) database searched for "query OAT1"47). OAT4 is a 551-amino-acid protein with 12 putative membrane-spanning domains. hOAT4 showed 38 to 44% identity with those of other members of the OAT family (Table 1). When expressed in Xenopus oocytes, OAT4 mediated the high-affinity transport of estrone sulfate (Km=1.01 µM) and dehydroepiandrosterone sulfate (Km=0.63 µM) in a sodium-independent manner (Table 2). OAT4 also mediated the transport of ochratoxin A and PAH. OAT4-mediated transport of estrone sulfate was inhibited by several sulfate conjugates, such as p-nitrophenyl sulfate, α-naphthyl sulfate, β-estradiol sulfate, and 4-methylumbelliferyl sulfate. In contrast, glucuronide conjugate showed little or no inhibitory effect on the OAT4-mediated transport of estrone sulfate. Recent report has shown that the transport mode of hOAT4 was also organic anion/dicarboxylate exchagner48). Northern blot analysis revealed that OAT4 mRNA is abundantly expressed in the placenta as well as in the kidney. OAT4 is the first member of the multispecific organic anion transporter family that is expressed abundantly in the placenta. OAT4 might be responsible for the elimination and detoxification of harmful anionic substances from the fetus.
fifth member of OAT (OAT5) was recently identified by Youngblood and Sweet from mouse kidney12). In 2001, human OAT5 have reported as hOAT4 by Sun et al.49). It was confirmed additionally as a renal steroid sulfate conjugate transporter50). The rat ortholog also reported in 200551). The mRNA of human, mouse and rat OAT5 was detected only kidney. When expressed in Xenopus oocytes, mOAT5 mediated the ochratoxin A (Km=2.0 µM), estrone sulfate (Km=2.2 µM) and dehydroepiandrosterone sulfate (Km=3.8 µM). rOAT5 also transported those substrate with sodium independently. The protein of OAT5 was localized at the apical membrane of renal proximal tubules.
The sixth member of OAT was recently identified from murine olfactory mucosa13). Based on sequence homology proposed to be an organic anion transporter; OAT6. However, they did not show any functional evidence. In this year, Schnabolk and coworkers have reported functional properties of OAT6 using X. laevis oocytes and transfected CHO cells52). Transport of estrone sulfate by mOat6 was demonstrated to be saturable having Km values of 109.8 µM in oocytes and 44.8 µM in CHO-mOat6 cells. This mOAT6-mediated estrone sulfate uptake was inhibited by 2,4-dichlorophenoxyacetate, salicylate, probenecid, and penicillin G. Accumulation of estrone sulfate mediated by mOat6 was significantly trans-stimulated by glutarate indicating that mOAT6 functions as an organic anion/dicarboxylate exchanger.
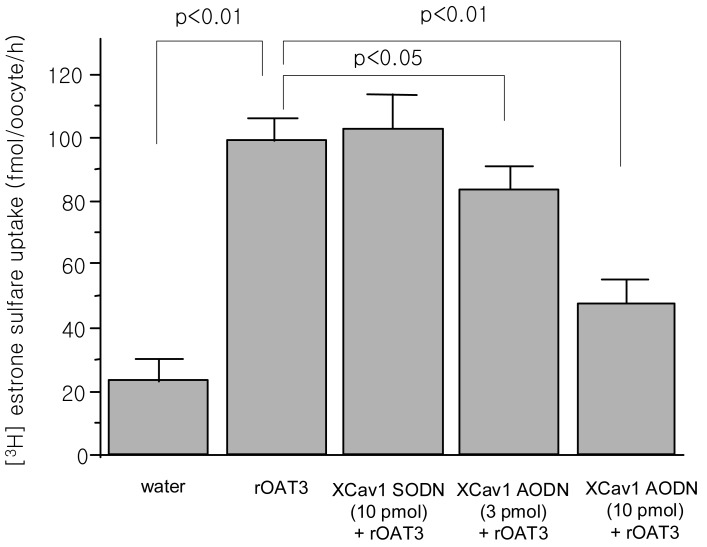
In recent decades, the caveolins have been reported as being important modulating molecules that bind to some proteins that have biological functions. Caveolins are integral membrane proteins that are present in caveolae, the small flask-shaped and detergent insoluble invaginations in the plasma membrane (Fig. 3), and they have been implicated as functioning in the vesicular transport processes. Recent studies have shown that several transporter proteins including p-glycoprotein 53), cationic amino acid transporter54), glucose transporter55), and sodium-calcium exchanger56) are localized in caveolin-enriched lipid raft microdomains and they are regulated by caveolins. The caveolin-binding motif is a protein-protein interaction and is composed of eight to nine amino acid residues : øXøXXXXø or øXXøXXXXø (where X is any amino acid, and ø designates aromatic amino acids like Trp (W), Phe (F) or Tyr (Y) and X can be any amino acids). The scanning of rOAT1 and rOAT3 amino acid sequence showed several caveolin-binding motifs within the transmembrane domain or near the intracellular surface of the plasma membrane. Recently Kwak et al. have reported that rOAT1 and rOAT3 proteins were existed in caveolae enriched fraction isolated by sucrose gradient ultracentrifugation from rat kidneys57, 58). The proteins of OATs and caveolins were co-precipitated by each respected antibodies (Fig. 4). When the rOAT1 and rOAT3 cRNA were expressed with antisense oligodeoxynucleotide of caveolins, the transport ability of rOAT1 or rOAT3 was decreased (Fig. 5). These results show that functional properties was regulated by scaffolding protein as well as protein kinase C.
The past few years have witnessed great advances in our knowledge of the molecular pharmacology of renal organic anion transport. The organic anion transport system is also important as a drug or xenobiotics transport system. The techniques for molecular biology enabled us to obtain much information about the identity of the organic anion transporters. The mechanism of excretion of organic anions into the primary urine is unknown. Understanding the detailed transport mechanism and distribution of each transporter in the kidney will give us much clues about renal drug clearance, drug-drug interaction, drug targeting to the kidney, and xenobiotic- or drug-induced nephropathy.
Acknowledgement
This work was supported in part by grants from the Korea Research Foundation Grants (KRF-2004-202-C00373 and KRF-2005-003-E00042).
References
1. Meier PJ. Molecular mechanisms of hepatic bile salt transport from sinusoidal blood into bile. Am J Physiol. 1995; 269:G801–G812. PMID: 8572210.

2. Muller M, Jansen PL. Molecular aspects of hepatobiliary transport. Am J Physiol. 1997; 272:G1285–G1303. PMID: 9227463.

3. Moller JV, Sheikh MI. Renal organic anion transport system : pharmacological, physiological, and biochemical aspects. Pharmacol Rev. 1982; 34:315–358. PMID: 6763702.
4. Ullrich KJ, Rumrich G. Renal transport mechanisms for xenobiotics : chemicals and drugs. Clin Investig. 1993; 71:843–848.

5. Pritchard JB, Miller DS. Mechanisms mediating renal secretion of organic anions and cations. Physiol Rev. 1993; 73:765–796. PMID: 8415925.

6. Sperber I. Secretion of organic anions in the formation of urine and bile. Pharmacol Rev. 1959; 11:109–134. PMID: 13633440.
7. Weiner I, Mudge GH. Renal tubular mechanisms for excretion of organic acids and bases. Am J Med. 1964; 36:743–762. PMID: 14141450.

8. Ullrich KJ, Rumrich G. Contraluminal transport systems in the proximal renal tubule involved in secretion of organic anions. Am J Physiol. 1988; 254:F453–F462. PMID: 3354682.

9. Pritchard JB, Miller DS. Comparative insights into the mechanisms of renal organic anion and cation secretion. Am J Physiol. 1991; 261:R1329–R1340. PMID: 1750557.

10. Sekine T, Cha SH, Endou H. The multispecific organic anion transporter (OAT) family. Eur J Physiol. 2000; 440:337–350.

11. Marshall EKJ, Vicker JL. The mechanisms of the elimination of phenolsulphonphthalein by the kidney -a proof of secretion by the convoluted tubules. Bull Johns Hopkins Hosp. 1923; 34:1–7.
12. Youngblood GL, Sweet DH. Identification and functional assessment of the novel murine organic anion transporter Oat5 (Slc22a19) expressed in kidney. Am J Physiol. 2004; 287:F236–F244.

13. Monte JC, Nagle MA, Eraly SA, Nigam SK. Identification of a novel murine organic anion transporter family member, OAT6, expressed in olfactory mucosa. Biochem Biophys Res Commun. 2004; 323:429–436. PMID: 15369770.

14. Sweet DH, Wolff NA, Pritchard JB. Expression cloning and characterization of ROAT1. The basolateral organic anion transporter in rat kidney. J Biol Chem. 1997; 272:30088–30095. PMID: 9374486.
15. Uwai Y, Okuda M, Takami K, Hashimoto Y, Inui KI. Functional characterization of the rat multispecific organic anion transporter OAT1 mediating basolateral uptake of anionic drugs in the kidney. FEBS Lett. 1998; 438:321–324. PMID: 9827570.

16. Lu R, Chan BS, Schuster VL. Cloning of the human kidney PAH transporter : narrow substrate specificity and regulation by protein kinase C. Am J Physiol. 1999; 276:F295–F303. PMID: 9950961.
17. Takeda M, Sekine T, Endou H. Regulation by protein kinase C of organic anion transport dirived by rat organic anion transporter 3 (rOAT3). Life Sci. 2000; 67:1087–1093. PMID: 10954042.
18. Zhou f, Illsley np, You G. Functional characterization of a human organic anion transporter hOAT4 in placental BeWo cells. Eur J Pharm Sci. 2006; (in press).

19. Kuze K, Graves P, Leahy A, Wilson P, Stuhlmann H, You G. Heterologous expression and functional characterization of a mouse renal orgnic anion transporter in mammalian cells. J Biol Chem. 1999; 274:1519–1524. PMID: 9880528.
20. Shimada H, Moewes B, Burckhardt G. Indirect coupling to Na of p-aminohippuric acid uptake into rat renal basolateral membrane vesicles. Am J Physiol. 198; 253:F795–F801. PMID: 3120599.

21. Pritchard JB. Intracellular α-ketoglutarate controls the efficacy of renal organic anion trasnport. J Pharmacol Exp Ther. 1995; 274:1278–1284. PMID: 7562499.
22. Prichard JB. Coupled transport of p-aminohippurate by rat basolateral membrane vesicles. Am J Physiol. 1988; 255:F597–F604. PMID: 3177651.
23. Masereeuw R, Russel FGM, Miller DS. Multiple pathways of organic anion secretion in renal proximal tubule revealed by confocal microscopy. Am J Physiol. 1996; 271:F1173–F1182. PMID: 8997391.

24. Burckhardt G, Porth J, Wolff NA. Functional and molecular characterization of renal transporters for p-aminohippurate (PAH). Nova Acta Leopoldina. 1998; 78:35–40.
25. Hosoyamada M, Sekine T, kanai Y, Endou H. Molecular cloning and functional expression of a multispecific organic anion transporter from human kidney. Am J Physiol. 1999; 276:F122–F128. PMID: 9887087.
26. Race JE, Grassl SM, Willians WJ, Holtzaman EJ. Molecular cloning and characterization of two nevel human renal organic anion transporters (hOAT1 and hOAT3). Biochem Biophys Res Commun. 1999; 255:508–514. PMID: 10049739.
27. Wolff NA, Werner A, Burkhardt S, Burckhardt G. Expression cloning and characterization of a renal organic anion transporter from winter flounder. FEBS Lett. 1997; 417:287–291. PMID: 9409735.

28. Apiwattanakul N, Sekine T, Chaioungdua A, Kanai Y, Nakajima N, Sophasan S, Endou H. Transport properties of nonsteroidal anti-inflammatory drugs by organic anion transporter 1 expressed in Xenopus laevis oocytes. Mol Pharmacol. 1999; 55:847–854. PMID: 10220563.
29. Jariyawat S, Sekine T, Takeda M, Apiwattanakul N, Kanai Y, Sophasan S, Endou H. The interaction and transport of β-lactam antibiotics with the cloned rat renal organic anion transporter 1 (OAT1). J Pharmacol Exp Ther. 1999; 290:672–677. PMID: 10411577.
30. Wada S, Tsuda M, Sekine T, Cha SH, Kimura M, Kanai Y, Endou H. Rat multispecific organic anion transporter 1 (rOAT1) transports zidovudine, acyclovir, and other antiviral nucloside analogs. J Pharmacol Exp Ther. 2000; 294:844–849. PMID: 10945832.
31. Sekine T, Cha SH, Kanai Y, Endou H. Molecular biology of multispecific organic anion transporter family (OAT family). Clin Exp Nephrol. 1999; 3:237–243.

32. Ullrich KJ. Renal transporters for organic anions and organic cations, structure requirements for substrantes. J Membrane Biol. 1997; 158:95–107. PMID: 9230087.
33. Tsuda M, Sekine T, Takeda M, Cha SH, Kanai Y, Kimura M, Endou H. Transport of ochratoxin A by renal multispecific organic anion transporter 1. J Pharmacol Exp Ther. 1999; 289:1301–1305. PMID: 10336520.
34. Endou H. Recent advances in molecular mechanisms of nephrotoxicity. Toxicol Lett. 1998; 102-103:29–33. PMID: 10022228.

35. Burckhardt BC, Wolff NA, Burckhardt G. Electrophysiological characterization of an orgnic anion transporter cloned from winter flounder kidney (fROAT). J Am Soc Nephrol. 2000; 11:9–17. PMID: 10616835.
36. Tojo A, Sekine T, Nakajima N, Hosoyamada M, Kanai Y, Kimura K, Endou H. Immunohistochemical localization of multispecific renal organic anion transporter 1 in rat kidney. J Am Soc Nephrol. 1999; 10:464–471. PMID: 10073596.

37. Nakajima N, Sekine T, Cha SH, Tojo A, Hosoyamada M, Kanai Y, Yan K, Awa S, Endou H. Develpmental changes in multispecific organic anion transporter 1 (OAT1) expression in the rat kidney. Kidney Int. 2000; 57:1608–1616. PMID: 10760096.
38. Kim GH, Na KY, Kim SY, Joo KW, Oh Y, Chae SW, Endou H, Han JS. Up-regulation of organic anion transporter 1 protein is induced by chronic furosemie or hydrochlorothiazide infusion in rat kidney. Nephrol Dial Transplant. 2003; 18:1505–1511. PMID: 12897087.
39. Simonson GD, Vincent AC, Roberg KJ, Huang Y, Iwanij V. Molecular cloning and characterization of a novel liver-specific transport protein. J Cell Sci. 1994; 107:1065–1072. PMID: 8056831.

40. Sekine T, Cha SH, Tsuda M, Apiwattanakul N, Nakajima N, Kanai Y, Endou H. Identification of multispecific organic anion transporter e expressed predominantly in the liver. FEBS Lett. 1998; 429:179–182. PMID: 9650585.
41. Kojima R, Sekine T, Kawachi M, Cha SH, Suzuki Y, Endou H. Immunolocalization of multispecific organic anion transporters, OAT1, OAT2, and OAT3, in rat kidney. J Am Soc Nephrol. 2002; 13:848–857. PMID: 11912243.

42. Kobayashi Y, Ohshiro N, shibusawa A, Sasaki T, Tokuyama S, Sekine T, Endou H, Yamamoto T. Isolation, characterization and differential gene expression of multispecific organic anion transporter 2 in Mice. Mol Pharm. 2002; 62:7–14.

43. Cha SH, Kim HP, Jung NH, Lee WK, Kim JY, Cha YN. Down-regulation of organic anion transporter 2 mRNA expression by nitric oxide in primary cultured rat hepatocytes. IUBMB Life. 2002; 54:129–135. PMID: 12489640.

44. Kusuhara H, Sekine T, Utsunomiya-Tate N, Tsuda M, Kojima R, Cha SH, Sugiyama Y, kanai Y, Endou H. Molecular cloning and characterization of a new multispecific organic anion transporter from rat brain. J Biol Chem. 1999; 274:13675–13680. PMID: 10224140.

45. Sweet DH, Chan LM, Walden R, Yang XP, Miller DS, Pritchard JB. Organic anion transporter 3 (Slc22a8) is a dicarboxylate exchanger indirectly coupled to the Na+ gradient. Am J Physiol. 2003; 284:F763–F769.
46. Cha SH, Sekine T, Fukushima J, Kanai Y, Kobayashi Y, Goya T, Endou H. Identification and characterization of human organic anion transporter 3 expressing predominantly in the kidney. Mol Pharmacol. 2001; 59:1277–1286. PMID: 11306713.

47. Cha SH, Sekine T, Kusuhara H, Yu E, Kim JY, Kim DK, Sugiyama Y, Kanai Y, Endou H. Molecular cloning and characterization of multispecific organic anion transporter 4 expressed in the placenta. J Biol Chem. 2000; 275:4507–4512. PMID: 10660625.

48. Ekaratanawong S, Anzai N, Jutabha P, Miyazaki H, Noshiro R, Takeda M, Kanai Y, Sophasan S, Endou H. Human organic anion transporter 4 is a renal apical organic anion/dicarboxylate exchanger in the proximal tubule. J Pharmacol Sci. 2004; 94:297–304. PMID: 15037815.
49. Sun W, Wu RR, van Poelje PD, Erion MD. Isolation of a family of organic anion transporters from human liver and kidney. Biochem Biophys Res Commun. 2001; 283:417–422. PMID: 11327718.

50. Kwak JO, Kim HW, Oh KJ, Ko CB, Park H, Cha SH. Characterization of mouse organic anion transporter 5 as a renal steroid sulfate transporter. J Steroid Biochem Mol Biol. 2005; 97:369–375. PMID: 16150593.

51. Anzai N, Jutabha P, Enomoto A, Yokoyama H, Nonoguchi H, Hirata T, Shiraya K, He X, Cha SH, Takeda M, Miyazaki H, Sakata T, Tomita K, Igarashi T, Kanai Y, Endou H. Functional characterization of rat organic anion transporter 5 (Slc22a19) at the apical membrane of renal proximal tubules. J Pharmacol Exp Ther. 2005; 315:534–544. PMID: 16079298.

52. Schnabolk GW, Youngblood GL, Sweet DH. Transport of Estrone Sulfate by the Novel Organic Anion Transporter Oat6 (Slc22a20). Am J Physiol. 2006; (in press).

53. Cai C, Zhu H, Chen J. Overexpression of caveolin-1 increases plasma membrane fluidity and reduces P-glycoprotein function in Hs578T/Dox. Biochem Biophys Res Commun. 2004; 320:868–874. PMID: 15240128.

54. McDonald KK, Zharikov S, Block ER, Kilberg MS. A caveolar complex between the cationic amino acid transporter 1 and endothelial nitric-oxide synthase may explain the "arginine paradox". J Biol Chem. 1997; 272:31213–31216. PMID: 9395443.

55. Karlsson M, Thorn H, Parpal S, Stralfors P, Gustavsson J. Insulin induces translocation of glucose transporter GLUT4 to plasma membrane caveolae in adipocytes. FASEB J. 2002; 16:249–251. PMID: 11744627.

56. Bossuyt J, Taylor BE, James-Kracke M, Hale CC. Evidence for cardiac sodium-calcium exchanger association with caveolin-3. FEBS Lett. 2002; 511:113–117. PMID: 11821059.

57. Kwak JO, Kim HW, Oh KJ, Kim DS, Han KO, Cha SH. Co-localization and interaction of organic anion transporter 1 with caveolin-2 in rat kidney. Exp Mol Med. 2005; 37:204–212. PMID: 16000875.

58. Kwak JO, Kim HW, Song JH, Kim MJ, Park HS, Hyun DK, Kim DS, Cha SH. Evidence for rat organic anion transporter 3 association with caveolin-1 in rat kidney. IUBMB Life. 2005; 57:109–117. PMID: 16036570.

Fig. 1
Amino acid sequence and membrane topology of rat organic anion transporter 1. OATs show both N-terminus and C-terminus in intracellular site.

Fig. 2
Mechanisms of organic anion transport in renal tubular epithelial cells. Transepithelial movement of organic anions across basolateral or apical membrane is mediated by OATs.

Fig. 3
The structure of caveolae in plasmamembrane. The small flask shapes indicated by arrow were caveolae (from Vol. 46: H2222, Am J Physiol, 1999). Bar, 120 nm.
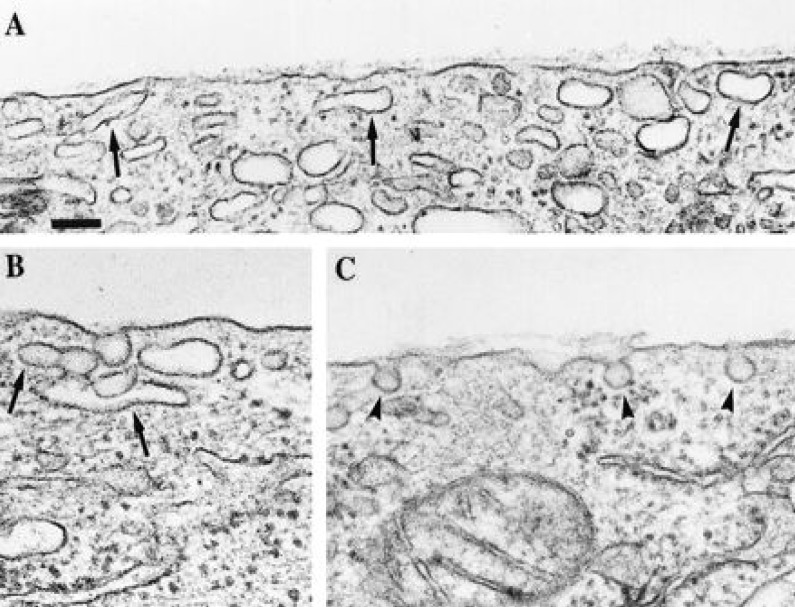
Fig. 4
Western blot analysis using isolated membrane fraction and immunoprecipitates by antibodies in the rat kidney. A) The kidneys were lysed using a teflon/glass homogenizer and a sonicator, and subjected to sucrose gradient centrifugation as described under Materials and Methods. B) Cell lysates were immuno-precipitated initially with antibodies of rOAT3 or Cav-1 and the respective immuno-precipitated proteins were loaded onto each lane of a 10% SDS-polyacrylamide gel. Kidney lysate was immuno-precipitated initially with rOA3 (left pannel) and was immuno-precipitated initially with Cav-1 antibodies (right pannel).
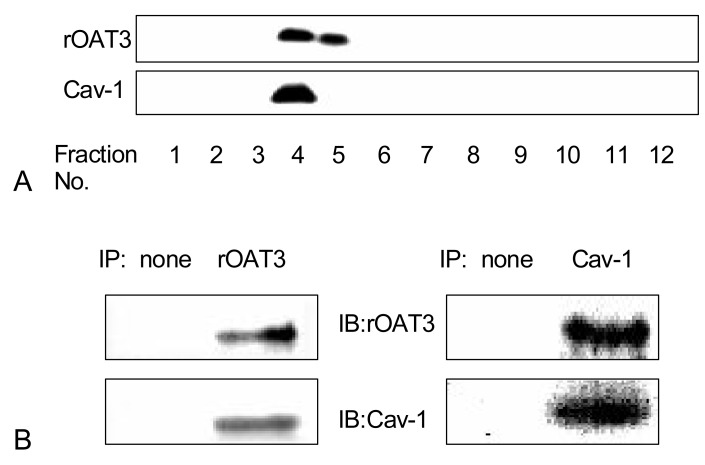
Fig. 5
The effects of Xenopus laevis sense or antisense oligodeoxynucleotide on rOAT3-mediated [3H] estrone sulfate uptake in Xenopus laevis oocyte expression system. Defolliculated stage VI and V oocytes were injected water, rOAT3 cRNA (20 ng/oocyte) with/without senese or antisense ODN (3 or 10 pmol/oocyte).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download