Introduction
The serum anion gap is a helpful parameter in the clinical diagnosis of various conditions. The commonest application of the anion gap is to classify cases of metabolic acidosis into those that do and those that do not have unmeasured anions in the plasma (Table 1). In this article, we briefly review the significance of the anion gap and the approach to the use of the serum anion gap.
Go to : 
Determinants of the serum anion gap
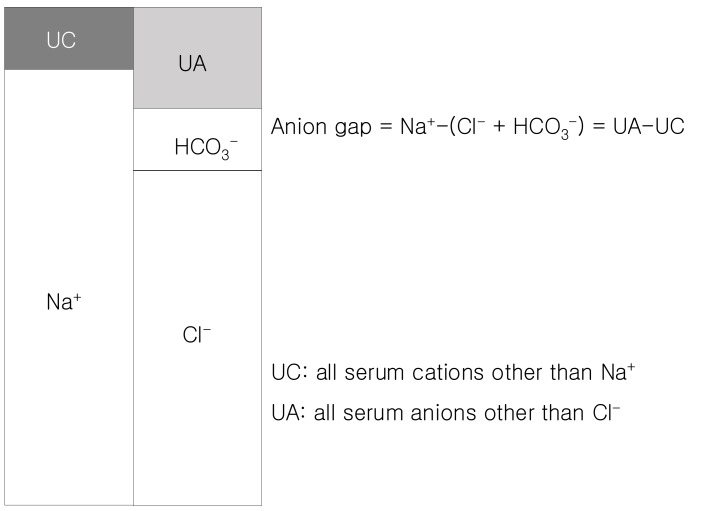
As charge balance precluded the existence of any gaps, the more accurate term should really be 'difference between unmeasured anions and unmeasured cations' which obviously lacks the brevity requisite for practical communication. Clinically, anion gap is equal to the difference between the plasma concentrations of the major cation (Na+) and the major measured anions (Cl-+HCO3-). It is important to understand that this entity actually equals [anionic proteins+inorganic phosphate+sulphate+organic anions]-[potassium+calcium+magnesium+cationic proteins] (Fig. 1)1). Since there are more unmeasured anions than unmeasured cations, the value of anion gap is usually positive. The classical value of a normal anion gap is considered 12±4 mEq/L, when sodium was determined by flame photometry (based on the principle of flame atomic emission spectrometry) and chloride by a colorimetric assay (mercuric-nitrate-thiocyanate colorimetric assay). Since the 1980s, ion-selective electrodes for specific ionic species were used for the measurement of serum electrolytes. The difference between the ionic concentration in the electrode (known) and the sample creates an electrical potential (measured) and the sample ionic concentration can be calculated. The measurement by ion-selective electrodes has caused a shift of the anion gap from 12±4 mEq/L down 6±3 mEq/L2). It is worthy for clinicians to understand the range of normal anion gap and the measuring methods for serum sodium and chloride in the laboratories that support their practice.
Go to : 
High anion gap
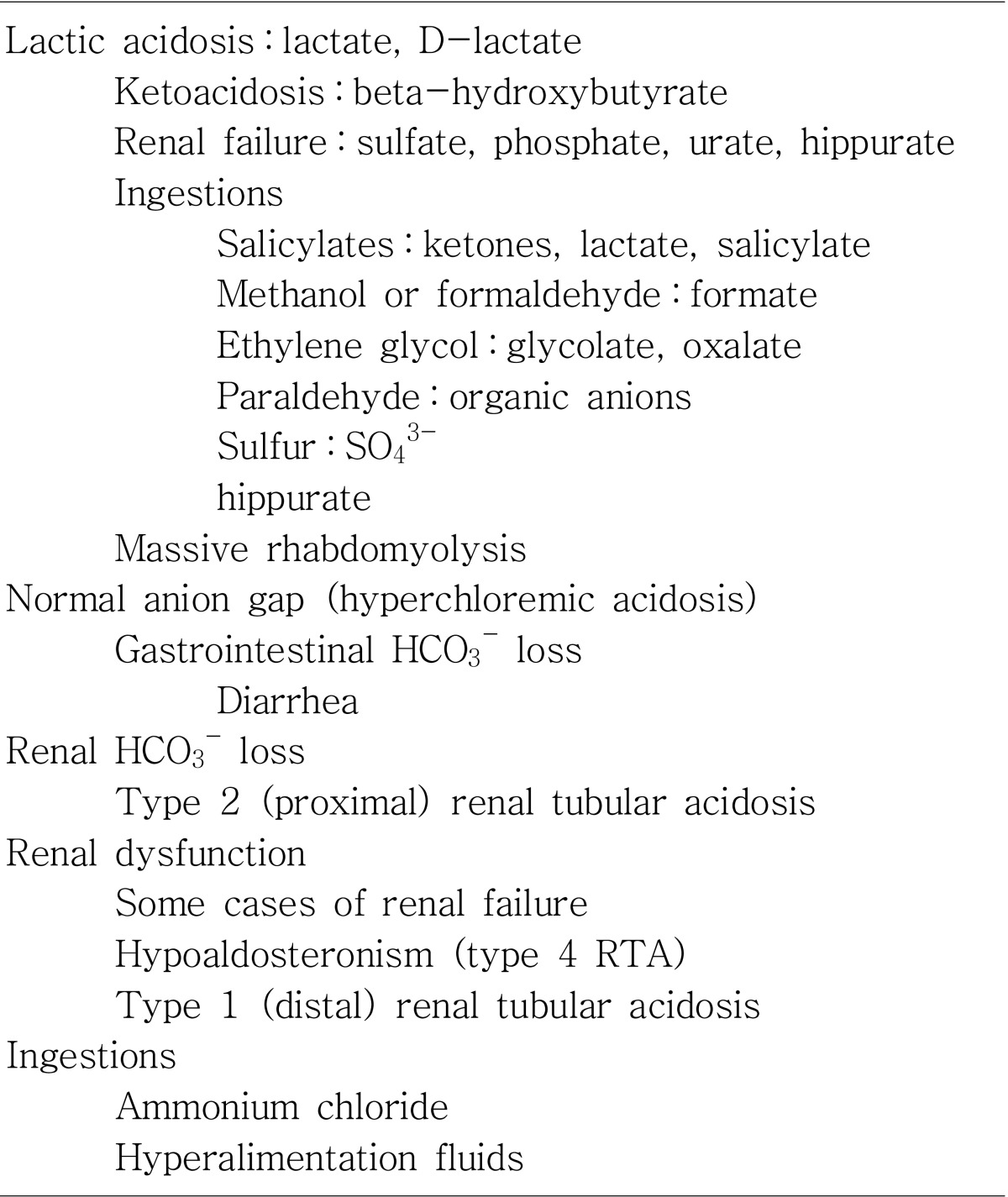
Why do we use the anion gap? It is important because an increased anion gap usually is caused by an increase in unmeasured anions, and that most commonly occurs when there is an increase in unmeasured organic acids, that is, an acidosis3, 4). Acids (eg, lactate and pyruvate) are protons donors and must be buffered by bicarbonate. The consumption of bicarbonate by the unmeasured anions will increase the anion gap by lowering the serum bicarbonate level. The total numbers of anions and cations are still equal, but the gap is increased because of a lowering of a measured anion, serum bicarbonate. The etiologies of an increase in organic acids have been well outlined. The most common ones can be remembered by the mnemonic MUDPILES : methanol, metformin, uremia, diabetic ketoacidosis, ethylene glycol, salicylates and starvation5, 6). These conditions produce an acid load that consumes bicarbonate, increases the anion gap, and lowers serum pH. If the patient is acidotic and has an elevated anion gap, it is almost certainly caused by one of these conditions, each one with us characteristic signs, symptoms, and laboratory values.
Go to : 
Low anion gap
Whereas the presence for a high anion gap educes the consideration of a differential diagnosis by reflex in daily clinical practice, a low anion gap often does not elicit the same warning to clinicians and hence often remains either undiscovered or neglected. The classical differential diagnosis of a low anion gap has changed since the ion-selective electrode has been introduced. A low anion gap has several utilities. First, it can be an early and sometimes only sign of an underlying disease process such as paraproteinemia. In addition to displacement of sodium-containing water from serum by large amounts of non-sodium-containing paraproteins, some paraproteins (eg, IgG in multiple myeloma) can have a net positive charge at physiological pH. This leads to an increase in unmeasured cations and a low anion gap7, 8). Concomitant severe hypercalcemia and hypoalbuminemia are often contributing factors to a low anion gap in multiple myeloma9). Since the only cation included in the anion gap calculation is sodium, severe hyperkalemia, hypercalcemia, hypermagnesemia or lithium intoxication theoretically can also lead to a significantly decreased anion gap. Second, at normal serum pH of 7.4, the majority of plasma proteins are anionic. Albumin with an average negative charge of 18 per mole at physiological pH has been shown to be responsible for approximately 75% of the unmeasured anions of the normal anion gap. A drop in albumin by 10 g/L therefore will cause the anion gap to fall by approximately 2.5 mEq/L at constant pH10, 11). Hypoalbuminemia is probably the commonest cause of a clinically relevant lowered anion gap. Third, a low anion gap can mask an underlying high anion gap acidosis and potentially delay intervention. While an increase in the anion gap is almost always caused by retained unmeasured anions, a decrease in the anion gap can be generated by multiple mechanisms.
Go to : 
Conclusion
In this short review, emphasis is placed on the fact that the serum anion gap is the difference between the unmeasured anions and the unmeasured cations, and any numerical analysis of this entity needs to take this equation into consideration. By exploring all the possible factors that can influence unmeasured cations and unmeasured anions can one truly extract useful information from the serum anion gap. It is advisable for the clinician to know the normal range of the anion gap and the assays used in measuring Na+ and Cl- in the laboratories supporting their practice.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download