The urea transporter family
The urea transporter family includes two main groups : UT-A and UT-B1). The four isoforms of rat UT-A (UT-A1-UT-A4) are found in the kidney, which are produced by alternate splicing of mRNA from a single gene2-4) UT-A1 is the longest isoform and the others are composed of part of UT-A1. UT- A2 is basically the C-terminal half of UT-A1, while UT-A3 is basically the N-terminal half of UT-A14, 5). UT-A4 basically consists of the N-terminal quarter of UT-A1 spliced to the C-terminal quarter of UT-A14). UT-A1 and UT-A3 are localized in the IMCD6-8), and UT-A2 is located in the descending thin limb of Henle's loop9,10). The precise tubular location of UT- A4 is unknown.
UT-B is encoded by a different gene from that encoding UT-A1 and was first cloned from a human bone marrow library11) and subsequently isolated from a rat inner medullary library by homology screening12,13). UT-B is expressed in the endothelial cells of the descending vasa recta and the membrane of red blood cells11-13).
Urine concentration
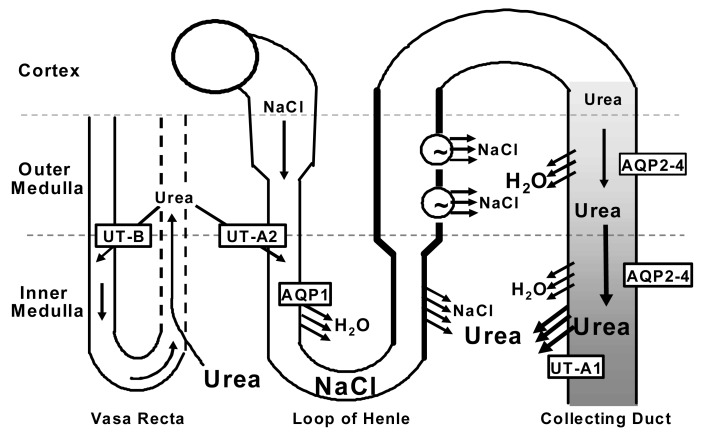
While NaCl is actively reabsorbed from the thick ascending limb in the outer medulla, there is no active process of NaCl reabsorption in the inner medulla. However, NaCl is reabsorbed from the thin ascending limb by virtue of the presence of urea in the inner medullary interstitium. The passive countercurrent multiplication explains the urine concentrating mechanism in mammals by which inner medullary tonicity is increased without active transport14, 15). In this theory, the higher urea concentration within the inner medullary interstitial fluid osmotically balances the higher NaCl concentration in the lumen of the thin ascending limb. Since the thin ascending limb is impermeable to water, highly permeable to NaCl, but only somewhat permeable to urea, NaCl is passively reabsorbed from the lumen of thin ascending limb to inner medullary interstitium by electrochemical gradient (Fig. 1).
Intra-renal urea recycling
The source of urea in the inner medullary interstitial fluid is reabsorption from the terminal inner medullary collecting duct. UT-A1 urea transporter is present in the terminal inner medullary collecting duct cells and facilitates the delivery of urea from collecting duct lumen to inner medullary interstitium. However, the highly concentrated urea in the terminal inner medulla could not be achieved if urea was rapidly taken up by the fenestrated ascending vasa recta and returned to the systemic circulation. UT-A2 in the descending thin limb of loops of Henle and UT-B in descending vasa recta enable a large fraction of the urea carried in the ascending blood to be reintroduced into the deep inner medulla. This creates an intra-renal urea recycling process that minimizes the loss of urea from the renal medulla and maintains the medullary osmotic gradient (Fig. 1).
Long term regulation of urea transporters
1. UT-A1
In Diabetes mellitus (DM), unabsorbed glucose retains water in the lumen and causes severe osmotic diuresis. In rats with uncontrolled DM (induced by streptozotocin), UT-A1 urea transporter and AQP2 water channel abundances in the collecting duct were significantly increased after 10-21 days after streptozotocin injection16-18), suggesting that these transporter proteins are up-regulated during DM-induced osmotic diuresis in order to prevent a serious volume depletion.
On the other hand, vasopressin rapidly increases the phosphorylation of UT-A119) and is known to be elevated in DM20, 21), which bring the thought that the increase of UT-A1 abundance in diabetic rats may be due to their high plasma vasopressin level.
To explore the effect of vasopressin on UT-A1 abundance in diabetic rats, Brattleboro rats (which lack circulating vasopressin) were infused with vasopressin or made diabetic by streptozotocin injection or received both procedures together22). DM for 10 days did not increase UT-A1 abundance in Brattleboro rats devoid of vasopressin, while vasopressin infusion (for 12 days) did significantly increase UT-A1 abundance, compared to untreated Brattleboro rats. DM combined with vasopressin infusion had more increase in UT-A1 abundance over vasopressin infusion alone. These results suggest that vasopressin is necessary for the increase of UT-A1 abundance during diabetes. It is also suggested that a factor other than vasopressin is responsible for the increase in UT-A1 protein abundance because the same amount of vasopressin was administered to the two vasopressin treated groups of Brattleboro rats22).
Urea is the major urinary solute in mammals, comprising 40-50% of total urinary solute in rats on a regular diet (containing 1% NaCl and 23% protein). Rats with streptozotocin-induced DM have an increase in absolute urea excretion, but the relative amount (percent) of urea in total urinary solute is actually decreased (to below 30%) due to the marked increase in glucose excretion17). The ongoing osmotic diuresis due to non-reabsorbable glucose causes water to be retained in the tubule lumen which dilutes the urine urea concentration. Several urea-specific signaling pathways have been identified in cultured mIMCD3 cells and the renal medulla23, 24), suggesting the possibility that changes in the percentage or concentration of urea in the urine or medullary interstitium could be a factor that regulates UT-A1 protein abundance.
To prove our hypothesis, diabetic rats were fed 10% urea diet in order to increase urea excretion, thereby keeping the percent of urea in total urinary solute at the control level. The urine urea concentration in this group was significantly increased when compared to that of diabetic rats with regular diet, although it was reduced when compared to control. UT-A1 abundance did not change in urea fed diabetic rats, which suggests that the increase of UT-A1 in diabetic rats is due to the decrease of urea in the urine25).
To further examine our hypothesis, rats were fed high salt diet containing 4% NaCl to induce NaCl diuresis. An additional group of rats were fed 4% NaCl and 3% urea in order to keep the percent of urea in total urinary solute at the control level. The absolute amount of urea excretion in the rats with 4% NaCl diet was not different from that of control rats, but the percent of urea in total urinary solute and urine urea concentration were significantly decreased due to the markedly increased NaCl excretion. UT-A1 abundance in the rats with 4% NaCl diet was significantly increased but not in the rats with 4% NaCl plus 3% urea diet25).
Next, we fed rats 5% urea diet in order to increase the percent of urea in total urinary solute (to over 60%) by increasing urea excretion. Usually, low urine urea concentrations and low urine osmolalities occur simultaneously, making it difficult to know which one is responsible for an increase in UT-A1 abundance. However, these two conditions can be dissociated if total urinary solute is increased by urea itself. In the rats with 5% urea diet, the urine urea concentration was increased, but the urine osmolality was decreased, which suggests that too much urea is not helpful for urine concentrating ability; rather it inhibits urine concentration by acting as an osmotic agent. Urea diuresis did not change UT-A1 abundance despite the decrease in urine osmolality and the presence of osmotic diuresis, suggesting that UT-A1 increases in response to a reduced percentage of urea in urine and/or a reduced urine urea concentration, rather than to a low urine osmolality25).
2. UT-A2 and UT-B
UT-A2 and UT-B mediate intra-renal urea recycling through the thin descending limb and descending vasa recta, respectively26, 27). UT-A2 is up-regulated in UT- B knock-out mice, suggesting that loss of one urea recycling pathway can be partially compensated for by increasing the other pathway28). Likewise, UT-B mRNA is up-regulated in UT-A2 knock-out mice, even though the increase was not significant29). UT-A2 protein or mRNA has been increased in dehydrated Sprague-Dawley rats3, 30) or DDAVP-infused Brattleboro rats9, 10), conditions where urine urea concentration is increased. UT-A2 and UT-B abundances were increased in urea diuresis25), where the urine urea concentration increased, but was unchanged or decreased in NaCl diuresis and DM-induced glucose diuresis18, 25), where the urine urea concentration is low. This suggests that UT-A2 and UT-B abundances increase when the medullary interstitial urea concentration is high, which would tend to increase intra-renal urea recycling during antidiuresis, thereby preventing the loss of urea from the medulla and maintaining medullary interstitial osmolality25).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download