Abstract
Backgrounds/Aims
Long-term immunosuppression regimens after liver transplantation (LT) are rarely reported in detail. We aimed to provide information on actual long-term immunosuppression regimens through this cross-sectional study.
Methods
Our institutional LT database was searched for adult patients who underwent primary LT operation from 2000 to 2016. We identified 3620 live recipients with actual information on immunosuppressive agent use for 1–17 years.
Results
The study cohort was divided into 7 groups according to posttransplantation period. The immunosuppressive agents used at the cross-sectional review period were tacrolimus in 2884 (79.7%), cyclosporine in 445 (12.3%), mycophenolate mofetil in 2007 (55.4%), and everolimus in 138 (3.8%) recipients. There was no marked difference in immunosuppressive agent use according to pretransplantation liver malignancy or type of LT operation. Tacrolimus, cyclosporine, mycophenolate mofetil, and everolimus were used in 97.4%, 1.8%, 60.9%, and 9.2%, respectively, in the year 2 group; 94.1%, 3.9%, 51.6%, and 8.3%, respectively, in the year 3 group; 87.3%, 8.4%, 68.9%, and 4.8%, respectively, in the year 4–5 group; 78.2%, 12.9%, 64.6%, and 3.0%, respectively, in the year 6–7 group; 76.9%, 10.8%, 58.8%, and 2.4%, respectively, in the year 8–10 group; 66.7%, 22.4%, 43.4%, and 1.5%, respectively, in the year 11–15 group; and 73.8%, 15.4%, 32.9%, and 1.7%, respectively, in the year ≥15 group.
Liver transplantation (LT) requires lifelong immunosuppression (IS) unless the patient acquires operational tolerance. Several kinds of immunosuppressive agents (ISAs) have been administered after LT, and every LT center usually has its own IS regimen protocols. Nearly all IS regimen protocols for LT include calcineurin inhibitor (CNI), mycophenolate mofetil (MMF), and steroid. Steroid dose is usually tapered off within short periods or intentionally omitted. Mammalian target of rapamycin (mTOR) inhibitor is increasingly administered as indicated for renal dysfunction or malignancy. IS regimen protocols are often summarized in published clinical studies from each institution; however, the complete details of institutional IS regimens are only occasionally reported.12 Especially for long-term IS regimens after LT, actual details are rarely reported.
The purpose of this study was to provide information on actual long-term IS regimens used in a high-volume LT center through a cross-sectional study in 3620 adult LT recipients.
This is a cross-sectional study on the actual long-term use of ISAs in adult LT recipients. We set the timing of cross-sectional review during 2 months from December 2017 to January 2018.
The LT database at our institution was searched to identify adult patients who underwent primary LT during 17 years from January 2000 to December 2016. The inclusion criteria were patient survival for 12 months after LT and until the end of December 2017, recipient age ≥18 years at LT operation, Korean ethnicity, and regular visits to the outpatient clinic of our institution. Finally, we identified 3620 live LT recipients with actual information on the administration of ISAs for 1–17 years. Our study protocol was approved by the institutional review board of our institution.
The peritransplantation primary IS protocols used for adult LT recipients at our institution consisted of interleukin-2 receptor inhibitor, intraoperative steroid bolus (5–10 mg/kg), intravenous or oral CNI and corticosteroid recycling beginning on day 1, and adjunctive MMF for patients showing CNI-associated adverse effects or for IS augmentation. For the control of CNI-associated adverse effects, tacrolimus and cyclosporine were occasionally exchanged. There were no differences in IS regimens between living-donor and deceased-donor LTs. Corticosteroid was rapidly tapered off within the first 3 months.
The target 12-hour trough concentration of tacrolimus was around 10–15 ng/ml for the first 1 month, 8–10 ng/ml within the first year, 5–8 ng/ml at 2–3 years, 5 ng/ml at 4–5 years, 3–5 ng/ml at 6–10 years, and 2–3 ng/ml after 10 years. When MMF was used for CNI sparing, the target tacrolimus concentration was reduced to half or less. The detailed target trough levels of tacrolimus with and without MMF relative to the posttransplantation period have been summarized previously.12
For MMF monotherapy, the target mycophenolic acid (MPA) level was set to at least 2–3 ng/ml and MMF dosage was adjusted according to the degree of renal dysfunction and MPA therapeutic drug monitoring (TDM).34
Concerning mTOR inhibitors, only everolimus is currently covered for LT recipients by the Korean social health insurance program. Its main indications at our institution include hepatocellular carcinoma (HCC) recurrence, de novo malignancy, and renal dysfunction. Intentional weaning off of all ISAs was not considered to date at our institution.
As the recipient conditions at the peritransplantation period are diverse, the IS regimens are also highly variable. Thus, we did not include IS regimens during the first 1 year in the present analysis.
We divided the 3620 recipients into 7 groups according to the posttransplantation period, as follows: second year (year 2 group), third year (year 3 group), fourth-fifth year (year 4–5 group), sixth-seventh year (year 6–7 group), eight-tenth year (year 8–10 group), tenth-fifteenth year (year 11–15 group), and beyond the fifteenth year (year ≥15 group).
We also divided the recipients into 2 groups according to the presence of liver malignancy at the time of LT: malignancy group and non-malignancy group. Primary liver malignancy included HCC and other incidentally detected liver malignancies in the explant livers (combined HCC-cholangiocarcinoma and neuroendocrine tumor).
Continuous variables are reported as means with standard deviation. Categorical variables were compared using the chi-square test. A p-value of <0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using SPSS (version 22; IBM, New York, NY, USA) and Statistica (version 6.0; StatSoft, Tulsa, OK, USA) software.
The numbers of LT recipients according to the posttransplantation period were 348 in the year 2 group (9.6%), 337 in the year 3 group (9.3%), 557 in the year 4–5 group (15.4%), 565 in the year 6–7 group (15.6%), 720 in the year 8–10 group (19.9%), 853 in the year 11–15 group (23.6%), and 240 in the year ≥15 group (6.6%). The clinical profiles of these 7 groups are summarized in Table 1.
The ISAs used during the overall study period were tacrolimus in 2884 (79.7%), cyclosporine in 445 (12.3%), MMF in 2007 (55.4%), and everolimus in 138 (3.8%) recipients (Fig. 1).
In the year 2 group (n=348), the ISAs used were tacrolimus in 339 (97.4%), cyclosporine in 6 (1.8%), MMF in 212 (60.9%), and everolimus in 32 (9.2%) recipients, implicating that tacrolimus-MMF (n=199, 57.2%) and tacrolimus monotherapy (n=108, 31.0%) were the most used regimens.
In the year 3 group (n=337), the ISAs used were tacrolimus in 317 (94.1%), cyclosporine in 13 (3.9%), MMF in 174 (51.6%), and everolimus in 28 (8.3%) recipients, implicating that tacrolimus-MMF (n=160, 47.5%) and tacrolimus monotherapy (n=129, 38.3%) were the most used regimens and tacrolimus-everolimus use (n=27, 8.0%) was increased.
In the year 4–5 group (n=557), the ISAs used were tacrolimus in 486 (87.3%), cyclosporine in 47 (8.4%), MMF in 384 (68.9%), and everolimus in 27 (4.8%) recipients, implicating that tacrolimus-MMF (n=331, 59.4%) and tacrolimus monotherapy (n=129, 23.2%) were the most used regimens and the uses of tacrolimus-everolimus (n=24, 4.3%) and MMF monotherapy (n=24, 4.3%) were increased.
In the year 6–7 group (n=565), the ISAs used were tacrolimus in 442 (78.2%), cyclosporine in 73 (12.9%), MMF in 365 (64.6%), and everolimus in 17 (3.0%) recipients, implicating that tacrolimus-MMF (n=276, 48.8%) and tacrolimus monotherapy (n=149, 26.4%) were the most used regimens and the use of MMF monotherapy (n=50, 8.8%) was increased.
In the year 8–10 group (n=720), the ISAs used were tacrolimus in 554 (76.9%), cyclosporine in 78 (10.8%), MMF in 423 (58.8%), and everolimus in 17 (2.4%) recipients, implicating that tacrolimus-MMF (n=295, 41.0%) and tacrolimus monotherapy (n=242, 33.6%) were the most used regimens and the use of MMF monotherapy (n=88, 12.2%) was increased.
In the year 11–15 group (n=853), the ISAs used were tacrolimus in 569 (66.7%), cyclosporine in 191 (22.4%), MMF in 370 (43.4%), and everolimus in 13 (1.5%) recipients, implicating that tacrolimus monotherapy (n=361, 42.3%) and tacrolimus-MMF (n=197, 23.1%) were the most used regimens and the use of MMF monotherapy (n=91, 10.7%) was increased.
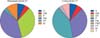
In the year ≥15 group (n=240), the ISAs used was tacrolimus in 177 (73.8%), cyclosporine in 37 (15.4%), MMF in 79 (32.9%), and everolimus in 4 (1.7%) recipients, implicating that tacrolimus monotherapy (n=134, 55.8%) was the most used regimen, whereas the uses of cyclosporine monotherapy (n=24, 10.0%), tacrolimus-MMF (n=39, 16.3%), and MMF monotherapy (n=26, 10.8%) were similar. These patterns of ISA use are collectively depicted in Fig. 2.
Pretransplantation malignancy was diagnosed in 1809 recipients (n=50%). The ISAs used were tacrolimus in 1436 (79.4%), cyclosporine in 227 (12.5%), MMF in 1003 (55.4%), and everolimus in 105 (5.8%) recipients in the malignancy group, and tacrolimus in 1448 (80.0%), cyclosporine in 218 (12.0%), MMF in 1004 (55.4%), and everolimus in 33 (1.8%) recipients in the non-malignancy group (Fig. 3). There was no difference in ISA use between these 2 groups except for everolimus (p<0.001).
Deceased-donor LT was performed in 396 recipients, in whom the ISAs used were tacrolimus in 305 (77.0%), cyclosporine in 41 (11.9%), MMF in 220 (55.6%), and everolimus in 12 (3.0%). On the other hand, in 3224 living-donor LT recipients, the ISAs used were tacrolimus in 2579 (80.0%), cyclosporine in 398 (12.3%), MMF in 1787 (55.4%), and everolimus in 126 (3.9%) (Fig. 4). The ISA use was very similar between these 2 groups (p≥0.73).
The results of this study revealed the changing trends in the use of IS regimens during >10 years, providing important insights into the recent changes in IS regimens and long-term ISA use at our institution.
Tacrolimus is nearly completely replacing cyclosporine. The proportion of cyclosporine use was 15.4% in the year ≥15 group, but it was gradually reduced and finally lowered to 1.8% in the year 2 group. Currently, cyclosporine is administered only when tacrolimus is intolerable because of serious adverse effects. Previously, cyclosporine was preferentially prescribed to recipients with hepatitis C virus infection for weak background reasons;5 however, the recent introduction of potent direct-acting antiviral agents has led to a change in such a preference.6789
One of the most recognizable results was the highly sustained proportion of MMF use. In the year 2 group, MMF was administered to 60.9% of patients, mostly combined with tacrolimus for CNI sparing. This seems natural because a majority of our recipients received CNI-MMF during the first year. Over time, the proportion of CNI-MMF changed primarily depending on the status of renal function. When the recipient well tolerated CNI, MMF dose was gradually tapered off during subsequent years. Adjunctive MMF use in the subtherapeutic TDM range seems to be unnecessary if the CNI TDM concentration is sufficiently high. Meanwhile, nephrotoxicity associated with CNI or of unknown origin developed, and the CNI use was gradually lowered or tapered off toward MMF monotherapy. MMF monotherapy was occasionally attempted before the first 2 or 3 years. It was used in 4.3% in the year 4–5 group; thereafter, its percentage of use increased to around 10%. The recipients administered with MMF monotherapy were meticulously selected after long-term observation and frequent MPA TDM; thus, nearly no patients who received MMF monotherapy experienced acute rejection. Considering that most of these recipients undergoing MMF monotherapy had renal dysfunction, meticulous adjustment of the MMF dosage is necessary because renal dysfunction interferes with the metabolism and excretion of MPA.1234
The introduction of mTOR inhibitors has caused some changes in ISA selection. Because everolimus began to be covered by social health insurance only in early 2016 in Korea, it is not widely used yet; thus, the proportion of mTOR inhibitor use in the pretransplantation malignancy group is still small. Thus far, we still do not yet include everolimus as a primary ISA during the early posttransplantation period. It is primarily administered after the development of HCC recurrence or de novo malignancy because mTOR inhibitors are known to be the only ISAs with anti-tumor effect.10111213141516 To date, everolimus monotherapy has been very rarely used at our institution because insurance policy permits combination with tacrolimus. We have intended to concurrently use mTOR inhibitor and sorafenib with the expectation of a synergistic effect against HCC recurrence; however, this was not demonstrated in clinical studies (unpublished data). In addition to the expected anti-tumor effect, we have attempted to use everolimus for its renal-sparing effect,1718 especially in recipients with poor absorption of MMF or those showing progressive deterioration of renal function despite long-term MMF monotherapy.
As far as we know, poor absorption of MMF is not an eligible indication for MMF monotherapy. We arbitrarily defined poor absorption as a 12-hour trough level of MPA of <1.0 mg/L after the administration of MMF 500 mg 2 times per day.3 As the potency of MMF for IS is not high enough,2 MMF monotherapy was rarely attempted within the first year of LT at our institution. Recently, the combination of MMF and everolimus together with very low-dose tacrolimus has been selectively attempted in recipients showing progressive renal dysfunction during the first year of LT.19202122 An analysis of high-volume data from the Scientific Registry of Transplant Recipients has revealed that mTOR inhibitor-based IS therapy is associated with improved survival after LT in recipients with HCC; however, it has also revealed a trend toward lower survival rates in non-HCC recipients.23
Changes in tablet dosage seem to be beneficial for patients taking multiple drugs daily. Concerning MMF, 1 original agent and 2 generic substitutions are concurrently used at our institution.2425 Generic double-dose (500 mg) MMF tablet provides convenience to recipients administered with high-dose MMF. Generic reduced-dose (0.25 mg) tacrolimus is very efficacious for recipients who had passed >5–10 years after LT, as well as for recipients with renal dysfunction because it enables meticulous adjustment of drug dosage. A considerable proportion of LT recipients require much smaller amounts of tacrolimus after a long period has passed than during the early posttransplantation period. In our clinical practice, we found that a higher proportion of recipients prefer taking tacrolimus 0.25 mg twice per day than tacrolimus 0.5 mg once per day probably because they are well accustomed to this medication style. Considering that a majority of LT recipients take various other drugs daily because of other concurrent diseases such as hypertension, diabetes mellitus, and hyperlipidemia, the actual advantage of once-daily tacrolimus seemed to be lower than expected in the current Korean setting.2627
The risk of acute rejection decreases as the posttransplantation period passes; however, subclinical pathological abnormalities emerge in a non-negligible number of recipients even after 10 years. Thus, the role of late liver biopsy after 10 years in LT recipients seems to be a matter of concern.282930 It is still unknown whether augmented IS can prevent or reduce the risk of such subclinical pathological abnormalities; however, we believe that a too low IS dose is not beneficial even after posttransplantation 10 years. Thus, we believe that it is better to continually maintain the IS regimen after posttransplantation 10 years. After 10 years of LT, around 20% of our recipients still receive the tacrolimus-MMF combination instead of CNI alone. A considerable proportion of these patients may be eligible for CNI monotherapy; however, we also believe that a combination of very low-dose tacrolimus and low-dose MMF is beneficial to prevent de novo malignancy.
Thus far, MMF is known to have a neutral effect on HCC recurrence.23 Recently, it was reported that MMF monotherapy is associated with a lower risk of cancer in LT recipients compared with maintenance IS with CNIs.31 In fact, MMF is an ISA with potential anti-cancer activity. MMF inhibits tumor cell growth and angiogenesis in vitro, although this effect has not translated to clinical anti-cancer benefit.32 In a single-center study, the combination of CNI and MMF in kidney transplant recipients was associated with a higher risk of malignancy than were other IS regimens.33 In contrast with these results, multicenter studies have shown that renal, cardiac, and liver transplant recipients who take MMF have a lower or at least not higher risk of malignancy than those without MMF therapy.343536
We identified that the long-term IS regimen was not different between recipients of deceased-donor and living-donor LTs. In fact, pretransplantation HCC was more common in living-donor LT recipients, whereas renal dysfunction was more common in deceased-donor LT recipients. We presume that these differences in patient characteristics led to the reciprocal offsetting of the differences in ISA selection.
The present study has several limitations. First, this is a retrospective single-center study with a cross-sectional review covering a short-term period. Second, we did not analyze the TDM concentration of each ISA and the intra-individual changes of ISAs because we have presented these findings previously.1234 Third, we did not separately present the profiles of patients who were alive at the time of data collection after the development of HCC recurrence or de novo malignancy because they will be presented in future reports of ongoing studies. The strong point of this study is that there is no case with lost data because all study patients are alive and regularly followed up at our institution.
In conclusion, we consider tacrolimus and MMF as the primary ISAs after LT and the indications for mTOR inhibitor have started to increase. We believe that our results will help establish tailored long-term IS regimen protocols in new or small-volume LT centers.
Figures and Tables
Fig. 1
Proportions of immunosuppressive regimens in 3620 liver transplant recipients. C, cyclosporine; M, mycophenolate mofetil; F, tacrolimus; T, everolimus. Combination of these capital letters indicates combination therapy.
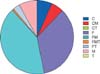
Fig. 2
Changes in immunosuppressive regimens relative to posttransplantation period. C, cyclosporine; M, mycophenolate mofetil; F, tacrolimus; T, everolimus. Combination of these capital letters indicates combination therapy.

Fig. 3
Proportions of immunosuppressive regimens in liver transplant recipients with or without pretransplantation liver malignancy. C, cyclosporine; M, mycophenolate mofetil; F, tacrolimus; T, everolimus. Combination of these capital letters indicates combination therapy.

ACKNOWLEDGEMENTS
This study was financially supported by the Organ Transplantation Center of Asan Medical Center and University of Ulsan cooperative system fund 2015-1518 (Chong Kun Dang Pharmaceutical Corp.).
References
1. Kang SH, Hwang S, Ha TY, Song GW, Jung DH, Kim KH, et al. Tailored long-term immunosuppressive regimen for adult liver transplant recipients with hepatocellular carcinoma. Korean J Hepatobiliary Pancreat Surg. 2014; 18:48–51.

2. Hwang S, Lee SG, Ahn CS, Kim KH, Moon DB, Ha TY, et al. A clinical assessment of mycophenolate drug monitoring after liver transplantation. Clin Transplant. 2010; 24:E35–E42.

3. Park YH, Hwang S, Song GW, Jung DH, Ahn CS, Kim KH, et al. Correlation between mycophenolic acid blood level and renal dysfunction in stable liver transplant recipients receiving mycophenolate monotherapy. Transplant Proc. 2014; 46:811–815.

4. Hwang S, Song GW, Jung DH, Park GC, Ahn CS, Moon DB, et al. Intra-individual variability of mycophenolic acid concentration according to renal function in liver transplant recipients receiving mycophenolate monotherapy. Ann Hepatobiliary Pancreat Surg. 2017; 21:11–16.

5. Sugawara Y, Kaneko J, Makuuchi M. Cyclosporin a for treatment of hepatitis C virus after liver transplantation. Transplantation. 2006; 82:579–580.

6. Lionetti R, Calvaruso V, Piccolo P, Mancusi RL, Mazzarelli C, Fagiuoli S, et al. Sofosbuvir plus daclatasvir with or without ribavirin is safe and effective for post-transplant hepatitis C recurrence and severe fibrosis and cirrhosis: a prospective study. Clin Transplant. 2018; 32.

7. Dharancy S, Coilly A, Fougerou-Leurent C, Duvoux C, Kamar N, Leroy V, et al. Direct-acting antiviral agent-based regimen for HCV recurrence after combined liver-kidney transplantation: Results from the ANRS CO23 CUPILT study. Am J Transplant. 2017; 17:2869–2878.

8. Abaalkhail F, Elsiesy H, Elbeshbeshy H, Shawkat M, Yousif S, Ullah W, et al. Treatment of patients with hepatitis C virus infection with ledipasvir-sofosbuvir in the liver transplant setting. Transplantation. 2017; 101:2739–2745.

9. Saxena V, Khungar V, Verna EC, Levitsky J, Brown RS Jr, Hassan MA, et al. Safety and efficacy of current direct-acting antiviral regimens in kidney and liver transplant recipients with hepatitis C: Results from the HCV-TARGET study. Hepatology. 2017; 66:1090–1101.

10. Huynh H, Ngo VC, Koong HN, Poon D, Choo SP, Thng CH, et al. Sorafenib and rapamycin induce growth suppression in mouse models of hepatocellular carcinoma. J Cell Mol Med. 2009; 13:2673–2683.

11. Yamanaka K, Petrulionis M, Lin S, Gao C, Galli U, Richter S, et al. Therapeutic potential and adverse events of everolimus for treatment of hepatocellular carcinoma - systematic review and meta-analysis. Cancer Med. 2013; 2:862–871.

12. Navarro-Villarán E, Tinoco J, Jiménez G, Pereira S, Wang J, Aliseda S, et al. Differential antitumoral properties and renal-associated tissue damage induced by tacrolimus and mammalian target of rapamycin inhibitors in hepatocarcinoma: in vitro and in vivo studies. PLoS One. 2016; 11:e0160979.

13. Zheng JF, Lu J, Wang XZ, Guo WH, Zhang JX. Comparative metabolomic profiling of hepatocellular carcinoma cells treated with sorafenib monotherapy vs. sorafenib-everolimus combination therapy. Med Sci Monit. 2015; 21:1781–1791.

14. Cholongitas E, Goulis I, Theocharidou E, Antoniadis N, Fouzas I, Giakoustidis D, et al. Everolimus-based immunosuppression in liver transplant recipients: a single-centre experience. Hepatol Int. 2014; 8:137–145.

15. Cholongitas E, Mamou C, Rodríguez-Castro KI, Burra P. Mammalian target of rapamycin inhibitors are associated with lower rates of hepatocellular carcinoma recurrence after liver transplantation: a systematic review. Transpl Int. 2014; 27:1039–1049.

16. Gomez-Martin C, Bustamante J, Castroagudin JF, Salcedo M, Garralda E, Testillano M, et al. Efficacy and safety of sorafenib in combination with mammalian target of rapamycin inhibitors for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2012; 18:45–52.

17. Fischer L, Klempnauer J, Beckebaum S, Metselaar HJ, Neuhaus P, Schemmer P, et al. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation--PROTECT. Am J Transplant. 2012; 12:1855–1865.

18. Rodríguez-Perálvarez M, Germani G, Darius T, Lerut J, Tsochatzis E, Burroughs AK. Reducing early exposure to calcineurin inhibitors: the key factor for a successful renal sparing strategy. Am J Transplant. 2013; 13:239.

19. Jiménez-Pérez M, González Grande R, Rando Muñoz FJ, de la Cruz Lombardo J, Muñoz Suárez MA, Fernández Aguilar JL, et al. Everolimus plus mycophenolate mofetil as initial immunosuppression in liver transplantation. Transplant Proc. 2015; 47:90–92.

20. Bilbao I, Dopazo C, Castells L, Lazaro J, Caralt M, Sapisochin G, et al. Immunosuppression based on everolimus in liver transplant recipients with severe early post-transplantation neurotoxicity. Transplant Proc. 2014; 46:3104–3107.

21. Beckebaum S, Cicinnati VR, Radtke A, Kabar I. Calcineurin inhibitors in liver transplantation - still champions or threatened by serious competitors? Liver Int. 2013; 33:656–665.

22. Herzer K, Strassburg CP, Braun F, Engelmann C, Guba M, Lehner F, et al. Selection and use of immunosuppressive therapies after liver transplantation: current German practice. Clin Transplant. 2016; 30:487–501.

23. Toso C, Merani S, Bigam DL, Shapiro AM, Kneteman NM. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology. 2010; 51:1237–1243.

24. Namgoong JM, Hwang S, Ahn CS, Kim KH, Moon DB, Ha TY, et al. A pilot study on the safety and efficacy of generic mycophenolate agent as conversion maintenance therapy in stable liver transplant recipients. Transplant Proc. 2013; 45:3035–3037.

25. Kim JM, Kwon CH, Yun IJ, Lee KW, Yu HC, Suh KS, et al. A multicenter experience with generic mycophenolate mofetil conversion in stable liver transplant recipients. Ann Surg Treat Res. 2014; 86:192–198.

26. Cassuto E, Pageaux GP, Cantarovich D, Rostaing L, Loupy A, Roche B, et al. Adherence to and acceptance of once-daily tacrolimus after kidney and liver transplant: results from OSIRIS, a French observational study. Transplantation. 2016; 100:2099–2106.
27. Trunečka P. Once-daily tacrolimus in liver transplantation: a ‘me-too drug’, or a therapeutic advantage. Curr Opin Organ Transplant. 2017; 22:118–122.
28. Ekong UD. The long-term liver graft and protocol biopsy: do we want to look? What will we find? Curr Opin Organ Transplant. 2011; 16:505–508.
30. Mells G, Mann C, Hubscher S, Neuberger J. Late protocol liver biopsies in the liver allograft: a neglected investigation? Liver Transpl. 2009; 15:931–938.

31. Aguiar D, Martínez-Urbistondo D, D'Avola D, Iñarrairaegui M, Pardo F, Rotellar F, et al. Conversion from calcineurin inhibitor-based immunosuppression to mycophenolate mofetil in monotherapy reduces risk of de novo malignancies after liver transplantation. Ann Transplant. 2017; 22:141–147.

32. Koehl GE, Wagner F, Stoeltzing O, Lang SA, Steinbauer M, Schlitt HJ, et al. Mycophenolate mofetil inhibits tumor growth and angiogenesis in vitro but has variable antitumor effects in vivo, possibly related to bioavailability. Transplantation. 2007; 83:607–614.

33. Wimmer CD, Rentsch M, Crispin A, Illner WD, Arbogast H, Graeb C, et al. The janus face of immunosuppression - de novo malignancy after renal transplantation: the experience of the Transplantation Center Munich. Kidney Int. 2007; 71:1271–1278.

34. Robson R, Cecka JM, Opelz G, Budde M, Sacks S. Prospective registry-based observational cohort study of the long-term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant. 2005; 5:2954–2960.

35. Lake JR, David KM, Steffen BJ, Chu AH, Gordon RD, Wiesner RH. Addition of MMF to dual immunosuppression does not increase the risk of malignant short-term death after liver transplantation. Am J Transplant. 2005; 5:2961–2967.

36. O'Neill JO, Edwards LB, Taylor DO. Mycophenolate mofetil and risk of developing malignancy after orthotopic heart transplantation: analysis of the transplant registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2006; 25:1186–1191.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download