Abstract
Hemobilia is a rare cause of upper gastrointestinal tract bleeding. Most cases are iatrogenic following medical interventions, most commonly liver biopsy and transhepatic cholangiography. We present a case of arteriobiliary fistula between the right hepatic artery and the common hepatic duct, in a case of Mirrizi syndrome, following endoscopic biliary stenting and presenting with hemobilia. The patient was treated by surgical disconnection of the fistula, ligation of the right hepatic artery, and bilioenteric anastomosis.
Hemobilia, defined as bleeding in the biliary tree, is a rare cause of upper gastrointestinal bleeding. Two thirds of cases are iatrogenic resulting from medical interventions, most commonly liver biopsy and transhepatic cholangiography.1 Endoscopic interventions including cannulation of the bile duct with guide wires and placement of metallic stents can also result in immediate and delayed bleeding.12 We present a case of arteriobiliary fistula between the right hepatic artery and the common hepatic duct following endoscopic biliary stenting and presenting with hemobilia.
A 36 year old male first presented to our hospital in October 2015 with symptoms of fever with chills and right hypochondriac pain. He had a history of 3 sessions of endoscopic retrograde cholangiopancreaticography (ERCP), common bile duct (CBD) stone extraction, and biliary stenting over a six week period. These were followed by laparoscopic cholecystectomy for cholelithiasis with choledocholithiasis in September of 2015. The plastic stent placed in the bile duct by ERCP was subsequently removed 10 days before the patient's presentation to our hospital.
On examination the patient's vital parameters were stable. Per abdomen examination revealed deep tenderness in the epigastrium. Scars from laparoscopic cholecystectomy had healed. Investigations revealed leukocytosis (total leukocyte count 13.5×103) with elevated serum bilirubin and liver enzymes (total bilirubin 5.91 mg/dl, direct bilirubin 5.37 mg/dl, aspartate transaminase 149 U/L, alanine transaminase 232 U/L, alkaline phosphatase 239 U/L, gamma-glutamyl transpeptidase 1109 U/L) suggesting obstructive jaundice with cholangitis. Magnetic resonance cholangiopancreaticography (MRCP) revealed a 13×7 mm-sized residual stone in the mid CBD with proximal bile duct dilatation (Fig. 1). The distal CBD was normal. The patient was admitted for an ERCP which showed thick pus coming from the CBD with a mid CBD stone. Attempts to extract the CBD stone failed. Two 7-Fr plastic stents were placed in the CBD to drain it. The patient was treated with intravenous antibiotics and hydration, and improved with the above measures. He was discharged at his request and was asked to return a week later for a repeat ERCP and bile duct clearance.
He did not return for follow up, but presented on 9 April 2016 at a private hospital in another city with another attack of cholangitis. A repeat ERCP with CBD stent change was performed at that hospital (Fig. 2). An attempt to remove the CBD stone was unsuccessful. At 48 hours after procedure, the patient developed rectal bleeding and was treated with intravenous antibiotics and fluids.
He subsequently presented at our hospital on 26 April 2016 for definitive clearance of the CBD stone. On presentation the patient's vital parameters were stable. Per abdomen examination was unremarkable. Investigations revealed hemoglobin of 10.4 g/dL with mild derangement of liver function tests. ERCP, which was done under general anesthesia, revealed blood in the antrum and duodenum, and blood was seen coming out of the ampulla. Fluoroscopy revealed a migrated stent in the CBD. Balloon dilatation of the ampulla was done after cannulating the CBD, and the blocked stent was removed. Cholangiogram was not obtained because the CBD was full of blood. A 10-Fr plastic stent was inserted in the bile duct. The patient was immediately scheduled for angiography.
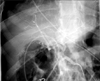
Selective celiac, superior mesenteric, hepatic and gastroduodenal artery angiogram was obtained. The visualized vessels and their branches appeared normal with no evidence of pseudoaneurysm or contrast extravasation (Fig. 3). A contrast-enhanced computerized tomography with angiogram also failed to reveal any aneurysm or contrast extravasation.
The patient was transfused with 2 units of blood and was hemodynamically stabilized. He was scheduled for surgery to remove the CBD calculi, to look for and deal with the source of bleeding, and to perform bilio-enteric diversion.
Analysis of the previously done MRCP and ERCP images clearly showed that the stone was in the low inserting cystic duct stump protruding into the mid common bile duct (i.e., Mirrizi syndrome). This could explain the failure to remove the CBD stone during the numerous ERCP attempts (Fig. 4).
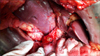
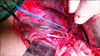
The patient was explored through a subcostal incision while under general anesthesia. Adhesions of the omentum to the liver were divided. The duodenum was freed from the retroperitoneum, and the peritoneum on the anterior surface of the hepatoduodenal ligament was cleared. The cystic duct stump with the stone in it, and the bile duct with the stent in it were identified. The cystic duct stump was opened and the stone removed (Fig. 5). Opening of cystic duct stump in to bile duct was identified and active oozing of blood from the bile duct was seen. The incision was extended in order to open the common bile duct, and the previously placed plastic stent was removed. Active ooze was seen coming from the proximal bile duct. In order to gain vascular control the common hepatic artery, the gastroduodenal artery, and the right hepatic artery were looped. The common bile duct was transected, and the proximal end lifted off the hepatic artery and portal vein. At the level of the common hepatic duct, a dense adhesion was found between the posterior surface of the hepatic duct and the anterior surface of the right hepatic artery (Fig. 6). The right hepatic artery, proximal and distal to this dense adhesion, was looped to gain vascular control (Fig. 6). A direct arteriobiliary fistula which was present at the site of the adhesion was disconnected. An attempt was made to repair the rent in the right hepatic artery, but because of inflamed, unhealthy tissue the segment of the artery was excised, and the stumps closed with a 5-0 polypropylene suture after confirming good backflow in the distal stump. The distal end of the bile duct was closed, and the proximal end anastomosed to a Roux-en-Y limb of jejunum to create a wide hepaticojejunostomy.
The patient had an uneventful postoperative course except for a superficial wound infection, and is asymptomatic at three months follow-up.
Hemobilia classically presents with Quinckes triad of bilary colic, jaundice and gastrointestinal bleeding; however the complete triad is seen in fewer than 40% of patients.3 Most cases occur after medical interventions such as percutaneous liver biopsy, transhepatic cholangiography, and ERCP guide wire and stent placements.12 In addition, blunt or penetrating liver trauma can cause hemobilia.1 Other causes include bile duct stones, biliary varices, benign and malignant tumors of the bile duct, liver surgery and transplantation, congenital or acquired vascular aneurysms and hepatitis.1245
The cause of hemobilia in our patient was multi-factorial. The patient had repeated episodes of cholangitis and multiple failed attempts of stone extraction and plastic stent placement. This must have led to the inflammatory adhesions which developed between the common hepatic duct and the right hepatic artery. The anatomical position of the bile duct and the right hepatic artery in the hepatoduodenal ligament facilitates formation of these adhesions.5 The ERCP guidewire, the attempt at stone extraction and stent placement may have led to an accidental puncture of the bile duct resulting in the formation of an arteriobiliary fistula. The occurrence of rectal bleeding within 48 hours of ERCP points towards this etiology. Case reports in the literature describe ERCP guide wire trauma related arteriobiliary and portobiliary fistula and plastic stent induced hemobilia.267 Once an arteriobiliary fistula develops, the patient develops immediate or delayed hemobilia as occurred in our patient.267
Direct visualization of the duodenal papilla confirms the diagnosis of hemobilia in this case. The priority is to localize and stop the bleeding. Selective angiography is the gold standard to identify and stop the bleeding by embolizing the offending vessel.12345 Angiography, however, failed to demonstrate the contrast leak in our patient, possibly because of the intermittent nature of the bleeding and the absence of a pseudoaneurysm. Because the patient had a large residual stone in the cystic duct stump protruding into the bile duct which caused recurrent jaundice, we proceeded with surgery. Bleeding from the bile duct after removal of the stone indicated some other cause of hemobilia. Further dissection of the bile duct identified the fistulous communication between the right hepatic artery and the common hepatic duct. The fistula was disconnected after proximal and distal arterial control was established, and the right hepatic artery was ligated. Good back flow was seen in the distal right hepatic artery stump, suggesting good collateral circulation, so that the risk of liver and bile duct ischemia was very low. Bilioenteric anastomosis was achieved using Roux-en-Y hepaticojejunostomy.
In conclusion, hemobilia due to hepatic arteriobiliary fistula is an extremely rare clinical entity. Identification of Mirizzi syndrome at the time of laparoscopic cholecystectomy may have prevented recurrent cholangitis, and the numerous ERCP interventions which led to development of the arteriobiliary fistula. More attention should be paid to the possibility of ERCP guide wire related complications. The use of newer, less traumatic guide wires and catheters for ERCP has likely reduced the frequency of iatrogenic hemobilia.1 Disconnection of the arteriobiliary fistula by endovascular embolization and surgery is the treatment of choice if an endovascular route fails.12
Figures and Tables
Fig. 1
Coronal magnetic resonance image showing 13 mm-sized stone in the mid common bile duct (white arrow).

Fig. 2
Endoscopic retrograde cholangiopancreaticography showing a filling defect in the mid common bile duct.
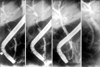
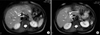
Fig. 4
T2-weighted magnetic resonance image showing stone (black shadow) in cystic duct stump protruding in to the common bile duct (A and B).
References
1. Baillie J. Hemobilia. Gastroenterol Hepatol (N Y). 2012; 8:270–272.
2. Nam JG, Seo YW, Hwang JC, Weon YC, Kang BS, Bang SJ, et al. Massive hemobilia due to hepatic arteriobiliary fistula during endoscopic retrograde cholangiopancreatography: an extremely rare guidewire-related complication. J Korean Soc Radiol. 2015; 72:348–351.
3. El Bouhaddouti H, Mazin K, Bouassria A, Mouaqit O, Benjelloun E, Ousadden A, et al. Hemobilia due to an iatrogenic arteriobiliary fistula complicating laparoscopic cholecystectomy: a case report. Open J Gastroenterol. 2014; 4:275–278.
4. Lin SZ, Tseng CW, Chen CC. Hepatic artery pseudoaneurysm presenting with Mirizzi syndrome and hemobilia. Clin Gastroenterol Hepatol. 2009; 7:e73.
5. Lee YT, Lin H, Chen KY, Wu HS, Hwang MH, Yan SL. Life-threatening hemobilia caused by hepatic pseudoaneurysm after T-tube choledochostomy: report of a case. BMC Gastroenterol. 2010; 10:81.
6. Kawakami H, Kuwatani M, Kudo T, Ehira N, Yamato H, Asaka M. Portobiliary fistula: unusual complication of wire-guided cannulation during endoscopic retrograde cholangiopancreatography. Endoscopy. 2011; 43:Suppl 2 UCTN. E98–E99.
7. Lee SH, Hong SG, Lee KY, Park PK, Kim SD, Lee M, et al. Delayed severe hemobilia after endoscopic biliary plastic stent insertion. Clin Endosc. 2016; 49:303–307.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download