Abstract
Backgrounds/Aims
Mirizzi syndrome (MS) is an uncommon complication of cholelithiasis. The aim of this study is to evaluate our 15-year experience in this challenging entity and to propose a new classification for this disease.
Methods
A retrospective study including patients diagnosed with Mirizzi syndrome and undergoing surgical procedures for Mirizzi syndrome between January 2000 and October 2015 was conducted. Data collected included clinical, surgical procedure, postoperative morbidity. Patients were evaluated according to the Csendes classification and the proposed system, in which patients were divided into three types and three subtypes.
Results
28 patients were included for analysis. They accounted as the 0.5% of a total of 4853 cholecystectomies performed in the study period. There were 21 women and 7 men. Initial laparotomic approach was performed in 12 patients and in 16 patients laparoscopic procedures were attempted. The procedure was completed in only 6 patients, 5 presenting type I and 1 type II Mirizzi syndrome. Mean postoperative stay was 15±9 days. Postoperative morbidity rate was 28%. Postoperative mortality was none.
First described by Kehr in 19051 and Ruge in 1908,2 Mirizzi syndrome or extrinsic biliary compression syndrome, is a rare complication of cholelithiasis.3 However, it was only in 1948, when Pablo Mirizzi published an article that established the eponym for this syndrome.45 Mirizzi syndrome is characterised by obstruction of the common bile duct (CBD), secondary to mechanical compression caused by the impaction of one or multiple gallstones in the neck of the gallbladder or cystic duct.6 The prevalence of this rare complication varies from 0.05 to 3%,78 and is more frequent in women.9
Over time, different classifications have been proposed for this syndrome. The most widely accepted classification is from McSherry et al.,10 published in 1982. It classifies Mirizzi syndrome into 2 types, based on endoscopic retrograde cholangiopancreatography (ERCP) findings: Type I involves extrinsic compression of the CBD, while in Type II, the cholecystobiliary fistula is already established. This classification was further expanded by Csendes et al.,11 who divided Type II into 3 subtypes (by thirds) based on the extent of bile duct wall involvement. A fifth type was added in 2007, which refers to any other type of Mirizzi syndrome associated with cholecystoenteric fistula (CEF).81213 Finally, in 2012, Beltrán14 established a new classification based on a suggestion by Solis-Caxaj,15 wherein the types of cholecystobiliary fistula are reduced to two, based on whether more or less than 50% of the CBD is affected.
Mirizzi syndrome is surgically treated, and is considered a challenge for all surgerons. As there are no signs or symptoms typical of this entity, and the percentage of patients diagnosed preoperatively varies widely, from 8% to 62.5%.16
Owing to the high incidence of surgical CBD injuries (SCBDI) described in operated patients with Mirizzi syndrome (up to 17%), both preoperative diagnosis and proper surgical planning are essential for the management of this disease.1417 Hence, the laparoscopic approach is considered controversial, and is only accepted in early stages of Mirizzi syndrome.1819
In this study, we retrospectively assess our experience in the diagnosis and treatment of Mirizzi syndrome. In order to standardize the surgical treatment, we propose a new classification of this syndrome.
Patients diagnosed with Mirizzi syndrome who underwent surgical procedures between January 2000 and October 2015, at the Hospital Doctor Peset in Valencia, Spain, were included in this retrospective study. The baseline characteristics evaluated were the patient's age, gender, and American Society of Anesthesiologists (ASA) grade. Data from the preoperative evaluation was collected, including symptoms at diagnosis and results of blood tests and radiological/endoscopic evaluation. Surgical records were obtained, including type of procedure, operating time, intraoperative complications, and need of conversion to open surgery. Postoperative morbidity included all complications that occurred during admission or in the 30 days following intervention. These data as well as other long-term events, were evaluated following the Dindo-Clavien classification.20 Binary data is presented as percentages. Continuous variables are presented as mean±standard deviation or median, and interquartile range (IQR) for non-normally distributed numerical variables.
Patients were evaluated according to the classification proposed by Csendes in 2007,13 and subsequently classified according to the following system proposed. Mirizzi syndrome would be classified as three types: Type 1 corresponds to the extrinsic compression of the bile duct; Type 2, when effect of the cholecystobiliary fistula is less than 50% of the CBD; and Type 3, when effect of the cholecystobiliary fistula is more than 50%. Within each of these sub-categories, Mirizzi syndrome is further classified according to three subtypes: Subtype A, in which no associated CEF is observed; Subtype B, in which the CEF is present without gallstone ileus; and Subtype C, in which the CEF is associated with gallstone ileus (Table 1 and Fig. 1).
A total of 28 patients with Mirizzi syndrome were included in this study. Of a total of 4853 cholecystectomies performed between January 2000 and October 2015 at our center, these patients represent a 0.5% incidence. Data from 21 (75%) women and 7 (25%) men was extracted.
The mean age was 64±17 years. A previous history of biliary disease was reported in 21 (75%) patients. Of these, 9 (32%) had presented episodes of biliary colic, 3 (11%) obstructive jaundice, 2 (7%) acute cholecystitis, 3 (11%) cholangitis, 1 (4%) presented with constitutional syndrome, 1 (4%) with gallstone ileus, whereas 3 patients (11%) had asymptomatic cholelithiasis. The median time from onset to surgery in patients who presented previous biliary disease was 4.5 months, with IQR 4.5-7 months. The most common signs and symptoms at diagnosis were abdominal pain and fever in 12 patients (43%). Laboratory findings and preoperative image diagnosis are shown in Table 2. Ultrasonpgraphy was performed in all patients, and was diagnostic in 14 of them. Preoperative diagnosis was obtained in 19 patients (68%), and 7 (25%) patients underwent ERCP prior to surgery; 5 patients (18%) were operated in an emergency setting due to a diagnosis of acute cholecystitis, and 23 patients (82%) underwent elective surgery. Laparotomy was performed in 12 patients (43%), and a laparoscopic approach was attempted in 16 (57%). The laparoscopic procedure could be completed in only 6 patients, of which 5 were type I, and only 1 was type II. The reasons for conversion in the remaining 9 cases were the presence of inflammatory mass and fibrosis that prevented the correct identification of structures within the Calot's triangle; in one case, the conversion was due to uncontrolled bleeding. Intraoperative cholangiography was carried out in 24 patients (86%).
In 1 patient who presented a cholecystobiliary fistula and associated CEF, Mirizzi syndrome type 1B, we opted to perform a cholecystectomy and choledochorrhaphy over T-tube placement and duodenorrhaphy. In another case of Mirizzi syndrome type 1C, CEF was associated with gallstone ileus, so we first treated the gallbladder ileus, followed by a second session where a laparoscopic cholecystectomy was performed.
A description of surgical procedures according the Csendes classification is shown in Table 2 and Fig. 2.
There was no postoperative mortality, and the morbidity rate was 28%. Postoperative biliary fistula was reported in 2 cases: one in an Mirizzi syndrome type I patient resulting from a cystic duct leak, and another in a type IV patient due to leakage of the hepaticojejunostomy; both were resolved with medical treatment (Dindo-Clavien grade I). One patient (Dindo-Clavien grade III) required urgent surgical review due to postoperative haemorrhage of the abdominal wall.
The mean postoperative stay was 15±9 days. The histopathology study revealed gallbladder adenocarcinoma in 3 patients (11%). In 2 cases, a palliative treatment was performed due to the age of patients. The third patient returned to his home country to complete the treatment.
Mean follow-up was 13±3.5 months. One type III patient who had been treated with a T-tube developed bile duct stenosis after 1 year that required a hepaticojejunostomy. Two patients were diagnosed with choledocholithiasis after 2 years, and were treated with ERCP.
Mirizzi syndrome is produced by an acute or chronic inflammation as a result of the impaction, or one or multiple gallstones, in the Hartman pouch, gallbladder infundibulum, or cystic duct. The pathophysiological sequence to formation of the cholecystobiliary fistula has been described by McSherry et al.10 and Csendes et al.,13 who also suggested that the appearance of CEF is the next stage in the same disease process. Predisposing factors for the development of this syndrome include the presence of a long cystic duct running parallel to the CBD, or a distal cystic duct insertion into the CBD.21 Its incidence in our setting was 0.5%, confirming the rarity of this syndrome, and is consistent with previous reports in developed countries.34567 Our results are also consistent with regards to both mean age at presentation (64 years old [53-70 years]),22 and higher frequency in women (76% of cases).9
At initial presentation and diagnosis, the most common symptom in our patients was abdominal pain associated with fever, followed by jaundice; 4 patients were asymptomatic at diagnosis.
No sensitive diagnostic tests are currently available for the diagnosis of Mirizzi syndrome. As in other biliary diseases, ultrasound is the first-line diagnostic test, with sensitivity varying from 8.3% to 62.7%15 (50% in our series). Ultrasound identifies various characteristic features of Mirizzi syndrome, such as presence of a small gallbladder, with thin walls containing one or multiple gallstones in the infundibulum, and dilatation of the intra- and extrahepatic bile ducts to the level of the obstruction. Occasionally, however, the only finding reported is the presence of choledocholithiasis.
The sensitivity of ultrasonography and computed tomography is similar, although the latter is more useful in ruling out neoplastic processes that affect the periportal region.23 Magnetic resonance cholangiopancreatography (MRCP) is a sensitive technique for detecting gallstones and bile duct stenosis, and can identify dilatation of the intrahepatic bile ducts, the degree of inflammation and presence of fistula, as well as differentiating tumour from inflammatory processes.24 Although it is a non-invasive, radiation-free test, it is not widely used due to its low availability and high cost.
ERCP is considered the gold standard for the diagnosis of Mirizzi syndrome,2526 with its sensitivity ranging from 55% to 90%.27 In addition to being a diagnostic procedure, it is also a therapeutic technique that enables extraction of gallstones from the CBD or biliary drainage using stents, allowing for elective surgery to be performed.28 Nevertheless, it presents some limitations. Fibrosis of the structures or the presence of choledocholithiasis sometimes makes it difficult to access the CBD; moreover, the technique is not free of complications, such as cholangitis or pancreatitis.
As previously noted, the intraoperative diagnosis rate is high in our series,29 so intraoperative cholangiography plays a fundamental role. This technique provides a definitive diagnosis, including the assessment of the presence and characteristics of potential biliary fistulas, defects in the CBD, and choledocholithiasis. It is extensively used in our hospital, and is systematically performed in patients with complex biliary disease, which explains the limited use of ERCP and MRCP (25% and 11%, respectively).
Based on the Csendes classification, type I in our study accounts for 39% of cases, and types II, III, IV and V for 21%, 14%, 14% and 10%, respectively (Table 2). However, the incidence of type I is higher in other published series, such as those presented by Cui et al.22 and Lledó et al.30 (Table 3). We believe this disparity can be explained because, in our setting, type I is often not identified as Mirizzi syndrome by the surgeon. Extrinsic compression of the CBD by an infundibular or cystic calculus is common in the context of acute cholecystitis. Although it can generally be treated adequately with cholecystectomy, the associated inflammatory process is usually the first step in the subsequent development of the syndrome, hence making the proper identification important. Accurate diagnosis is also important due to the high associated risk of SCBDI.
Different classifications for this syndrome have been proposed over time. In 2012, Beltrán14 proposed a new classification in order to simplify and enable standardized studies to be conducted. Fundamentally, it divides the cholecysto-biliary fistula into 2, rather than 3 types, based on whether more or less than 50% of the CBD wall is affected. In our opinion, this simplified classification establishes a standardised approximation to the surgical approach for this rare syndrome, indicating hepaticojejunostomy in the event that more than half of the wall is affected. Furthermore, as in the Csendes classification, it differentiates the type of Mirizzi syndrome in the presence or absence of CEF.
The main shortcoming of these classifications is that bile duct injuries associated with CEF, that significantly determines the course of treatment, are not markedly differentiated. These classifications do not classify the patients into two categories, namely with and without CEF.14 We believe that the presence of CEF should not constitute a type of Mirizzi syndrome, but a subtype within each of the type of Mirizzi syndrome. Therefore, we propose a new classification based on the classification suggested by Beltrán, and establish the following Mirizzi syndrome subtypes: A, no CEF; B, CEF without gallstone ileus; and C, CEF associated with gallstone ileus. It could thus be appropriate for guiding the treatment of both biliary and enteric damage (Fig. 3). Based on this classification, type IA in our study accounts for 39% of cases, types IB 4%, type IC 4%, type IIA 25% type IIB 4%, and type IIIA 25%. There were no cases of Mirizzi syndrome type IIC, IIIB and IIIC.
The laparoscopic approach to biliary lithiasis is currently the technique of choice. Nevertheless, although the preferred treatment for Mirizzi syndrome is surgical, the laparoscopic approach remains controversial owing to its intermediate success rate. Laparoscopy cannot be considered the procedure of choice in all cases,19 and SCBDI rates can reach up to 22%.16 In Mirizzi syndrome type 1A, the patients are reported to be successfully treated by laparoscopy, 18 although anterograde dissection is preferred in case of a major inflammatory component that prevents proper visualisation of the Calot's triangle.30 Subtotal cholecystectomy is an option to be considered in cases of severe fibrosis, due to its safety and excellent outcomes.731 Also, cases with associated choledocholithiasis can be treated during the same surgical session.32
According to our new proposed classification, the open approach is generally indicated for Mirizzi syndrome type 2A, by treating the bile duct defect by choledochorrhaphy over T-tube placement.92728 Subtotal cholecystectomies, primary choledochorrhaphies or choledocoplasties using gallbladder wall segments have also been described.22 When destruction of the CBD wall is greater, as in Mirizzi syndrome type 3A, biliary bypass (preferably hepaticojejunostomy) is the treatment of choice.182231 In Mirizzi syndrome subtype B associated with CEF without gallstone ileus, preference would be for open surgery, involving dissection and simple suture of the bilioenteric fistula over the implicated viscera wall, and treatment of the gallbladder. In 1 case in our series which presented a cholecystobiliary fistula and associated CEF, Mirizzi syndrome type 1B, we opted to perform a cholecystectomy and choledochorrhaphy over T-tube placement and duodenorrhaphy. In the other case of Mirizzi syndrome type 2B, we performed a choledocoduodenostomy after extending the solution of continuity of the fistula area, and cholecystectomy and primary choledochorrhaphy. For CEF associated with gallstone ileus (Mirizzi syndrome subtype C), two surgical options could be performed. Both conditions can be treated simultaneously, or the gallbladder ileus can be treated first, followed by subsequent treatment of the gallbladder in a second session, due to the deterioration and usually poor general health of these patients. A proposed by Beltrán,14 the two-step approach was our preferred option in the only type 1C patient in our series. This could explain why some Mirizzi syndromes are not diagnosed, as no action is subsequently taken on the bile duct. In any case, treatment of the CBD largely depends on the degree to which it is affected.
The postoperative mortality described in various series previously is less than 1%, as against nil in our series. However, morbidity varies widely, from 5%27 to 31%;30 Cui et al.22 estimate it to be around 9.5% (Table 3). We observed only 1 case of biliary fistula that resolved with conservative treatment, and 1 case of biliary stenosis that required surgical intervention. A close relationship exists between Mirizzi syndrome and gallbladder cancer, and between 0%22 and 28%33 of patients with a preoperative diagnosis of Mirizzi syndrome are reported to present gallbladder cancer. Both diseases share the same pathophysiological mechanism, namely the presence of gallstones for long durations, resulting in chronic inflammation and inducing damage to the bile duct mucosa.
In conclusion, Mirizzi syndrome today continues to be an entity with a very low incidence, and presents both a diagnostic and therapeutic challenge for surgeons. Our experience highlights the fact that although laparoscopic surgery plays a fundamental role in the treatment of various biliary diseases, its use in this syndrome is relegated to the very early stages. As many surgeons could be involved in treating this disease, this proposed classification could guide the surgical management of this syndrome and improve postoperative outcomes.
Figures and Tables
Fig. 1
Proposal for classification of Mirizzi syndrome of 3 types and 3 subtypes. Types: type 1, extrinsic compression of the bile duct; type 2, the cholecystobiliary fistula affects less than 50% of the common bile duct (CBD); and type 3, the cholecystobiliary fistula affects more than 50% of the CBD. Subtypes: A, no associated cholecystoenteric fistula (CEF); B, CEF is present without gallstone ileus; and C, CEF is associated with gallstone ileus.
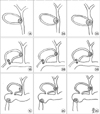
Fig. 2
Surgical treatment of 28 patients with Mirizzi syndrome. LC, laparoscopy cholecystectomy; OC, open complete cholecystectomy; OCS, open subtotal cholecystectomy; T, Tube placement; PCR, primary choledochorrhaphy; CD, choledocoduodenostomy; HJ Roux-en-Y, hepatico-jejunostomy.

Fig. 3
Classification proposal and surgical treatment of Mirizzi syndrome. LC, laparoscopy cholecystectomy; LCS, laparoscopy subtotalcholecystectomy; OC, open complete cholecystectomy; OCS, open subtotal cholecystectomy; T, Tube placement; PCR, primary choledochorrhaphy; CD, choledocoduodenostomy; HJ Roux-en-Y, hepatico-jejunostomy.

Table 2
Demographic, laboratory and surgery and morbidity data evaluated according to the classification proposed by Csendes13

ACKNOWLEDGEMENTS
The authors want to thank Dr. Domingo-del Pozo for his essential support in the performance of the present study.
References
1. Kehr H. Die in neiner klinik geubte technik de gallenstein operationen, mit einen hinweis auf die indikationen und die dauerersolge. Munchen: JF Lehman;1905.
2. Ruge E. Deitrage zur chirurgischen anatomie der grossen galenwege (Ductus hepaticus, choledochus, und pancreaticus). Arch Clin Chir. 1908; 78:47.
3. Abou-Saif A, Al-Kawas FH. Complications of gallstone disease: Mirizzi syndrome, cholecystocholedochal fistula, and gallstone ileus. Am J Gastroenterol. 2002; 97:249–254.
4. Mirizzi PL. Physiologic sphincter of the hepatic bile duct. Arch Surg. 1940; 41:1325–1333.
5. Mirizzi PL. Sindrome del conducto hepatico. J Int Chir. 1948; 8:731–777.
6. Pemberton M, Wells AD. The Mirizzi syndrome. Postgrad Med J. 1997; 73:487–490.
7. Dorrance HR, Lingam MK, Hair A, Oien K, O'Dwyer PJ. Acquired abnormalities of the biliary tract from chronic gallstone disease. J Am Coll Surg. 1999; 189:269–273.
8. Beltran MA, Csendes A, Cruces KS. The relationship of Mirizzi syndrome and cholecystoenteric fistula: validation of a modified classification. World J Surg. 2008; 32:2237–2243.
9. Zhong H, Gong JP. Mirizzi syndrome: experience in diagnosis and treatment of 25 cases. Am Surg. 2012; 78:61–65.
10. McSherry CK, Ferstenberg H, Virshup M. The Mirizzi syndrome: suggested classification and surgical therapy. Surg Gastroenterol. 1982; 1:219–225.
11. Csendes A, Díaz JC, Burdiles P, Maluenda F, Nava O. Mirizzi syndrome and cholecystobiliary fistula: a unifying classification. Br J Surg. 1989; 76:1139–1143.
12. Beltran MA, Csendes A. Mirizzi syndrome and gallstone ileus: an unusual presentation of gallstone disease. J Gastrointest Surg. 2005; 9:686–689.
13. Csendes A, Muñoz C, Albán M. Síndrome de Mirizzi-Fístula colecistobiliar, una nueva clasificación. Rev Chil Cir. 2007; 59:63–64.
14. Beltrán MA. Mirizzi syndrome: history, current knowledge and proposal of a simplified classification. World J Gastroenterol. 2012; 18:4639–4650.
15. Solis-Caxaj CA. Mirizzi syndrome: diagnosis, treatment and a plea for a simplified classification. World J Surg. 2009; 33:1783–1784.
16. Lai EC, Lau WY. Mirizzi syndrome: history, present and future development. ANZ J Surg. 2006; 76:251–257.
17. Baer HU, Matthews JB, Schweizer WP, Gertsch P, Blumgart LH. Management of the Mirizzi syndrome and the surgical implications of cholecystcholedochal fistula. Br J Surg. 1990; 77:743–745.
18. Erben Y, Benavente-Chenhalls LA, Donohue JM, Que FG, Kendrick ML, Reid-Lombardo KM, et al. Diagnosis and treatment of Mirizzi syndrome: 23-year Mayo Clinic experience. J Am Coll Surg. 2011; 213:114–119.
19. Antoniou SA, Antoniou GA, Makridis C. Laparoscopic treatment of Mirizzi syndrome: a systematic review. Surg Endosc. 2010; 24:33–39.
20. Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992; 111:518–526.
21. Zaliekas J, Munson JL. Complications of gallstones: the Mirizzi syndrome, gallstone ileus, gallstone pancreatitis, complications of “lost” gallstones. Surg Clin North Am. 2008; 88:1345–1368.
22. Cui Y, Liu Y, Li Z, Zhao E, Zhang H, Cui N. Appraisal of diagnosis and surgical approach for Mirizzi syndrome. ANZ J Surg. 2012; 82:708–713.
23. Cornud F, Grenier P, Belghiti J, Breil P, Nahum H. Mirizzi syndrome and biliobiliary fistulas: roentgenologic appearance. Gastrointest Radiol. 1981; 6:265–268.
24. Choi BW, Kim MJ, Chung JJ, Chung JB, Yoo HS, Lee JT. Radiologic findings of Mirizzi syndrome with emphasis on MRI. Yonsei Med J. 2000; 41:144–146.
25. Ravo B, Epstein H, La Mendola S, Ger R. The Mirizzi syndrome: preoperative diagnosis by sonography and transhepatic cholangiography. Am J Gastroenterol. 1986; 81:688–690.
26. Cruz FO, Barriga P, Tocornal J, Burhenne HJ. Radiology of the Mirizzi syndrome: diagnostic importance of the transhepatic cholangiogram. Gastrointest Radiol. 1983; 8:249–253.
27. Safioleas M, Stamatakos M, Safioleas P, Smyrnis A, Revenas C, Safioleas C. Mirizzi syndrome: an unexpected problem of cholelithiasis. Our experience with 27 cases. Int Semin Surg Oncol. 2008; 5:12.
28. Yonetci N, Kutluana U, Yilmaz M, Sungurtekin U, Tekin K. The incidence of Mirizzi syndrome in patients undergoing endoscopic retrograde cholangiopancreatography. Hepatobiliary Pancreat Dis Int. 2008; 7:520–524.
29. Tan KY, Chng HC, Chen CY, Tan SM, Poh BK, Hoe MN. Mirizzi syndrome: noteworthy aspects of a retrospective study in one centre. ANZ J Surg. 2004; 74:833–837.
30. Lledó JB, Barber SM, Ibañez JC, Torregrosa AG, Lopez-Andujar R. Update on the diagnosis and treatment of mirizzi syndrome in laparoscopic era: our experience in 7 years. Surg Laparosc Endosc Percutan Tech. 2014; 24:495–501.
31. Shah OJ, Dar MA, Wani MA, Wani NA. Management of Mirizzi syndrome: a new surgical approach. ANZ J Surg. 2001; 71:423–427.
32. Estellés Vidagany N, Domingo Del Pozo C, Peris Tomás N, Díez Ares JÁ, Vázquez Tarragón A, Blanes Masson F. Eleven years of primary closure of common bile duct after choledochotomy for choledocholithiasis. Surg Endosc. 2016; 30:1975–1982.
33. Redaelli CA, Büchler MW, Schilling MK, Krähenbühl L, Ruchti C, Blumgart LH, et al. High coincidence of Mirizzi syndrome and gallbladder carcinoma. Surgery. 1997; 121:58–63.
34. Aydin U, Yazici P, Ozsan I, Ersõz G, Ozütemiz O, Zeytunlu M, et al. Surgical management of Mirizzi syndrome. Turk J Gastroenterol. 2008; 19:258–263.
35. Kwon AH, Inui H. Preoperative diagnosis and efficacy of laparoscopic procedures in the treatment of Mirizzi syndrome. J Am Coll Surg. 2007; 204:409–415.
36. Kamalesh NP, Prakash K, Pramil K, George TD, Sylesh A, Shaji P. Laparoscopic approach is safe and effective in the management of Mirizzi syndrome. J Minim Access Surg. 2015; 11:246–250.
37. Xu XQ, Hong T, Li BL, Liu W, He XD, Zheng CJ. Mirizzi syndrome: our experience with 27 cases in PUMC hospital. Chin Med Sci J. 2013; 28:172–177.
38. Chowbey PK, Sharma A, Mann V, Khullar R, Baijal M, Vashistha A. The management of Mirizzi syndrome in the laparoscopic era. Surg Laparosc Endosc Percutan Tech. 2000; 10:11–14.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download