Abstract
Associating Liver Partition and Portal vein ligation for Staged hepatectomy (ALPPS) is a novel method to prevent post-hepatectomy hepatic failure. We present a case of periductal infiltrating intrahepatic cholangiocarcinoma undergone ALPPS, that was conducted as intraoperative choice instead of conducting preoperative portal vein embolization (PVE). A 65-year-old male patient was to undergo extended right posterior sectionectomy, but the operation plan was changed to conduct right hepatectomy with/without bile duct resection due to invasion of the right hepatic duct. After deciding to conduct ALPPS, we stopped further perihilar dissection and liver was transected. The right portal vein was ligated and Surgicel was densely packed between the transected hemilivers. There was rapid regeneration of the left liver on computed tomography follow-up, thus the second-stage right hepatectomy was conducted 10 days after the first-stage operation. Bile duct resection (BDR) was not performed due to heavy perihilar adhesion and inflammation, but fortunately tumor-negative bile duct resection margin was achieved after meticulous dissection. This patient recovered uneventfully and discharged nine days after the second-stage right hepatectomy. Thereafter he underwent concurrent chemoradiation therapy. He is doing well so far without evidence of tumor recurrence for 20 months after operation. In conclusion, this case suggests that ALPPS may be applied to an unexpected situation requiring PVE, but ALPPS is not recommend for treatment of perihilar malignancy requiring BDR.
Safe resection of the liver tumor has been one of the main focuses in the field of hepatobiliary surgery.1 To prevent post-hepatectomy hepatic failure, portal vein embolization (PVE) was first introduced.2 The next was staged operations including PVE or portal vein ligation.34 One of novel staged operations is Associating Liver Partition and Portal vein ligation for Staged hepatectomy (ALPPS).5 Its main indication is extensive bilobar colorectal liver metastases with small future remnant liver (FRL) volume.6789
We present a case of periductal infiltrating intrahepatic cholangiocarcinoma undergone ALPPS, that was conducted as an intraoperative choice instead of preoperative PVE.
A 65-year-old male patient admitted under the impression of intrahepatic cholangiocarcinoma. He had undergone colon resection twice two years and three years before admission, respectively. Preoperative imaging work-up led to diagnosis of intrahepatic cholangiocarcinoma of periductal infiltrating type (Fig. 1). Cancer antigen 19-9 was 23.4 U/ml and chorioembryonic antigen was 2.3 ng/ml. Indocyanine green retention test at 15 minutes was 14.5%. Other laboratory profiles were within normal limit. Colonoscopic study revealed no evidence of colon cancer recurrence.
We planned to conduct extended right posterior sectionectomy after evaluation of the extent of the intrahepatic duct involvement. Computed tomography (CT) volumetry revealed the left liver was approximately 35% of the total liver volume (TLV), thus we did not consider conducting of right hepatectomy.
During surgery, after partial resection of the right posterior section to expose the tumor-bearing area, the first-order branch of the right hepatic duct was invaded, thus being indicated for right hepatectomy with or without bile duct resection (BDR) instead of extended right posterior sectionectomy. After thorough examination of the left liver regarding quality and FRL volume, we conducted a two-stage operation instead of straightforward right hepatectomy with BDR.
After deciding to conduct ALPPS, we ceased further perihilar dissection to avoid tumor exposure and spread. Under temporary clamping of the right hepatic glissonian pedicle, the liver parenchyma was completely transected as in the right hepatectomy. And then, the right portal vein was meticulously dissected and ligated (Fig. 2). Ten pieces of Surgicel (ETHICON) were placed between the two hemilivers to facilitate later separation. Two sets of Jackson-Pratt drains were inserted to evacuate abdominal fluid.
Liver dynamic CT taken at four days after liver partition revealed rapid regeneration of the left liver. CT taken at eight days revealed that the FRL volume was 520 mL, which was 360 mL before operation (44% growth during eight days). Since hepatic parenchymal resection rate was lowered to approximately 50%, the second-stage operation was conducted 10 days after the first-stage liver partition and portal vein ligation (Fig. 3).
The brittle Surgicel was easily removed like minute sands and two hemilivers separated since there was no noticeable adhesion to the transected hepatic parenchyma. After ligating the right hepatic artery, the right hepatic duct was meticulously dissected because of heavy inflammatory changes at the perihilar area. First transection of the right hepatic duct revealed tumor-positive resection margin at the frozen-section biopsy. Second transection resulted in tumor-negative bile duct resection margin. Because of heavy perihilar inflammatory changes, we did not conduct additional BDR to minimize procedure-associated complications. Pathology reported that the tumor was moderately differentiated adenocarcinoma of periductal infiltrating type with perineural invasion, but no lymph node metastasis.
The patient recovered uneventfully from operation and discharged nine days after the second-stage right hepatectomy (Fig. 4). To reduce the risk of tumor recurrence, the patient underwent concurrent chemoradiation therapy. He is doing well so far without evidence of tumor recurrence for 20 months after operation.
ALPPS has recently been developed to induce rapid liver hypertrophy to reduce posthepatectomy liver failure. In 2012, Schnitzbauer et al.5 reported the technique of right portal vein ligation combined with in situ splitting, induces rapid left lateral liver lobe hypertrophy enabling two-staged extended right hepatic resection in small-for-size settings. In addition, de Santibañes and Clavien10 proposed the acronym ALPPS for this surgical technique of associating liver partition and portal vein ligation for staged hepatectomy.
ALPPS and portal vein embolization (PVE) share the same concept to induce contralateral liver hypertrophy after ipsilateral portal flow deprivation. There are some major differences, with different indications. The unique indication of ALPPS is multiple colorectal liver metastasis, in which metastasectomy was conducted at the FRL and liver partitioning of the removable liver at the first-stage operation. PVE can accelerate unwanted growth of metastastic tumors at the FRL, thus not being indicated. If concurrent BDR is anticipated like in this case, ALPPS may not be indicated because of tumor spread at the first-stage operation and technical difficulty from heavy perihilar inflammatory changes at the second-stage operation. Thus, so far, ALPPS has been usually conducted on patients with multiple colorectal liver metastases. Outcome of ALPPS for perihilar cholangiocarcinoma was unfavorable, in which 3-month mortality rate was 48% in comparison with 13% in control patients. Thus, ALPPS is not recommended for perihilar cholangiocarcinoma.1112 In contrast, PVE has been conducted to unilaterally located liver tumors such as hepatocellular carcinoma, intrahepatic cholangiocarcinoma as well as perihilar and gallbladder cancers requiring bile duct resection.
In this case, if we had evaluated the extent of tumor involvement more accurately as well as willing to resect more aggressively, we could conduct preoperative PVE because PVE can more easily prevent risk of post-hepatectomy hepatic failure. We conducted ALPPS instead of PVE because liver partition and portal vein ligation was readily available during laparotomy.
In ALPPS, maintenance of liver partition without adhesion is critical to facilitate the second-stage operation. Non-absorbable vinyl bag and similar ones have been used so far. In contrast, a considerable amount of Surgicel was used for such a purpose in this case. After 10 days, the Surgicel became brittle and was easily removed like sands, thus making two hemilivers separate easy. This method prevented liver adhesion as well as ensured hemostasis, thus being a readily available method to maintain liver partition.
There are two noticeable advancements in PVE to enhance efficacy. The first is additional hepatic vein embolization (HVE). We presented our experience of 42 cases of sequential PVE-HVE.13 Primary diseases were bile duct cancers, hepatocellular carcinomas, and intrahepatic cholangiocarcinoma and gallbladder cancer. These patients demonstrated insufficient FRL regeneration following PVE, thus HVE was conducted to induce further regeneration. No PVE-HVE procedure-associated complications occurred. In the bile duct cancer group, the degree of FRL hypertrophy was 13.3% after PVE, 28.9% after PHV-HVE, and 117.1% at two weeks after right hepatectomy. Thus, sequential application of HVE following PVE safely and effectively induces further FRL regeneration, especially in non-cirrhotic livers.1314 More recently, simultaneous PVE-HVE was equally safe and effective,15 but we think that sequential approach is more a reasonable option. We think that the liver-regenerating effect of PVE and HVE is quite comparable to that of partial ALPPS.
The second is PVE with contralateral installation of stem cells. It was presented to evaluate the clinical outcome of patients undergoing PVE and autologous CD133+ bone marrow-derived stem cell application before extended right hepatectomy, in which post hoc analysis revealed better survival for the PVE with stem cell group compared with the PVE group.16 Promising data from this survival analysis suggest that PVE, together with CD133+ bone marrow-derived stem cell pretreatment, could positively impact overall outcomes after extended right hepatectomy. Another study evaluated progress of FRL volume in patients with liver metastases after PVE with the application of hematopoietic stem cells, in which PVE with the application of hematopoietic stem cells significantly facilitates growth of FRL volume in comparison with PVE only.17 This method could be one of the new suitable approaches to increase the resectability of liver tumors.
In conclusion, this case suggests that ALPPS may be applied to an unexpected situation requiring PVE, but ALPPS is not recommend for treatment of perihilar malignancy requiring BDR.
Figures and Tables
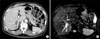 | Fig. 1Preoperative imaging study findings. (A) Liver computed tomography scan reveals ill-defined mass with dilatation of the right posterior hepatic duct; and (B) Magnetic resonance imaging reveals segmental dilatation of the right posterior hepatic duct. |
 | Fig. 2Intraoperative photographs. (A) Liver parenchyma was transected and the right portal vein was encircled with 1-0 black silk (arrow); and (B) Ligation of the right portal vein induces dark discoloration of the right liver. |
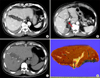 | Fig. 3Image findings after the first-stage operation. (A) Liver dynamic computed tomography (CT) taken at four days after liver partition revealed rapid regeneration of the left liver and packing of Surgicel between the transected hemilivers; (B) A 4-day CT image reveals complete deprivation of the right portal vein flow (an arrow indicates the site of portal vein ligation); (C) CT taken at eight days revealed further regeneration of the left liver; and (D) Gross photograph of the resected right liver specimen. |
References
1. Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007; 356:1545–1559.
2. Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990; 107:521–527.
3. Jaeck D, Oussoultzoglou E, Rosso E, Greget M, Weber JC, Bachellier P. A two-stage hepatectomy procedure combined with portal vein embolization to achieve curative resection for initially unresectable multiple and bilobar colorectal liver metastases. Ann Surg. 2004; 240:1037–1049.
4. Kianmanesh R, Farges O, Abdalla EK, Sauvanet A, Ruszniewski P, Belghiti J. Right portal vein ligation: a new planned two-step all-surgical approach for complete resection of primary gastrointestinal tumors with multiple bilateral liver metastases. J Am Coll Surg. 2003; 197:164–170.
5. Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012; 255:405–414.
6. Schadde E, Ardiles V, Robles-Campos R, Malago M, Machado M, Hernandez-Alejandro R, et al. Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg. 2014; 260:829–836. discussion 836-838.
7. de Santibañes E, Alvarez FA, Ardiles V. How to avoid postoperative liver failure: a novel method. World J Surg. 2012; 36:125–128.
8. Lau WY, Lai EC, Lau SH. Associating liver partition and portal vein ligation for staged hepatectomy: the current role and development. Hepatobiliary Pancreat Dis Int. 2017; 16:17–26.
9. Schadde E, Ardiles V, Slankamenac K, Tschuor C, Sergeant G, Amacker N, et al. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg. 2014; 38:1510–1519.
10. de Santibañes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure: the “ALPPS” approach. Ann Surg. 2012; 255:415–417.
11. Olthof PB, Coelen RJS, Wiggers JK, Groot Koerkamp B, Malago M, Hernandez-Alejandro R, et al. High mortality after ALPPS for perihilar cholangiocarcinoma: case-control analysis including the first series from the international ALPPS registry. HPB (Oxford). 2017; 19:381–387.
12. Lang H, de Santibanes E, Clavien PA. Outcome of ALPPS for perihilar cholangiocarcinoma: case-control analysis including the first series from the international ALPPS registry. HPB (Oxford). 2017; 19:379–380.
13. Hwang S, Ha TY, Ko GY, Kwon DI, Song GW, Jung DH, et al. Preoperative sequential portal and hepatic vein embolization in patients with hepatobiliary malignancy. World J Surg. 2015; 39:2990–2998.
14. Ahn JH, Kim HD, Abuzar SM, Lee JY, Jin SE, Kim EK, et al. Intracorneal melatonin delivery using 2-hydroxypropyl-β-cyclodextrin ophthalmic solution for granular corneal dystrophy type 2. Int J Pharm. 2017; 529:608–616.
15. Guiu B, Chevallier P, Denys A, Delhom E, Pierredon-Foulongne MA, Rouanet P, et al. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: the liver venous deprivation technique. Eur Radiol. 2016; 26:4259–4267.
16. am Esch JS, Schmelzle M, Fürst G, Robson SC, Krieg A, Duhme C, et al. Infusion of CD133+ bone marrow-derived stem cells after selective portal vein embolization enhances functional hepatic reserves after extended right hepatectomy: a retrospective single-center study. Ann Surg. 2012; 255:79–85.
17. Ludvík J, Duras P, Třeška V, Matoušková T, Brůha J, Fichtl J, et al. Portal vein embolization with contralateral application of stem cells facilitates increase of future liver remnant volume in patients with liver metastases. Cardiovasc Intervent Radiol. 2017; 40:690–696.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download