Abstract
Backgrounds/Aims
The protective effect of everolimus (EVR) in hepatocellular carcinoma (HCC) patients who receive liver transplantation in terms of reducing the recurrence has not been sufficiently investigated in clinical trials. In this second stage of our ongoing study, we intend to analyze the effects of EVR as an immunosuppressant, when it is started in the early phase after living donor liver transplantation (LDLT), on HCC recurrence in patients with HCC within the University of California at San Francisco (UCSF) criteria.
Methods
From January 2011 to June 2013, a total of 250 patients underwent LDLT for HCC at our institute. The patients with HCC within the UCSF criteria were included in the study and divided in two groups depending upon the postoperative immunosuppression. Group A: HCC patients that received EVR+TAC based immunosuppressive regimen (n=37). Group B: HCC patients that received standard TAC based immunosuppressive regimen without EVR (n=29). The target trough level for EVR was 3 to 5 ng/ml while for TAC it was 8–10 ng/ml.
Results
For group A patients, the mean trough level of the EVR was 3.47±1.53 ng/ml (range, 1.5–11.2) with a daily dose of 1.00±0.25 mg/day. For group A and B, the average TAC trough levels were 6.97±3.98 ng/ml (range, 2.50 to 11.28 ng/ml) and 6.93±2.58 (range, 2–16.30), respectively. The 1-year, 3-year and 4-year overall survival achieved for Group A patients was 94.95%, 86.48% and 86.48%, respectively while for Group B patients it was 82.75%, 68.96%, and 62.06%, respectively (p=0.0217).
Liver transplantation is an acceptable modality of treatment for unresectable HCC that falls within acceptable criteria such as the Milan criteria1 or the UCSF criteria2 with 5-year survival rates of >70%.3 In Asia, LDLT is a quick source of donor liver allografts for the waitlisted HCC patients and patients with end-stage-liver disease (ESLD) as the chances of getting deceased organs are dismal.4
Because of continued increase in the cohort population of HCC patients who require liver transplantation, many clinical studies in the recent era have described and evaluated the expanded criteria for HCC and the outcome of such patients after LDLT.56789 However, recurrence of HCC remains a problem even after LDLT, and the risk is higher for HCC recipients beyond the Milan criteria.10 Recurrence of HCC has been reported to occur in approximately 10–30% of liver transplant recipients over 5 years post transplantation among the patients within the Milan criteria, and the risk increases further among recipients of extended criteria organs.1011 The role of post-transplant immunosuppression in the recurrence of HCC as well as de novo malignancy in addition to renal dysfunction and hepatitis C virus (HCV) recurrence is well documented.1213 Promotion of tumor growth (adenocarcinoma) by both, cyclosporine and tacrolimus, through a non-immunologically mediated mechanism related to augmented transforming growth factor-beta production has been demonstrated in vitro and in animal studies.1415 High levels of calcineurin inhibitors (CNIs) in the early post-transplant period have been shown to be an independent risk factor for HCC recurrence.16
A potential role of mammalian target of rapamycin (mTOR) inhibitors in reducing the recurrence of various cancer types has been discussed recently.10 By definition, post transplantation tumor recurrence is the result of metastasis prior to or during surgery, and any possible effect of mTOR inhibitors when used as an immunosuppressive therapy in reducing the recurrence of HCC thus needs to be evaluated. The mTOR inhibitors inhibit angiogenesis in cancer cells by reduction of vascular endothelial growth factor (VEGF), which is expressed in excess in cancer cells. The apoptotic regulator Aakt, a serine-threonine kinase, is activated in many cancers, and its downregulation by m-TOR inhibitors has been found to interfere with tumor growth in various cancers as well as in HCC.17 EVR is now a commonly used mTOR inhibitor, and its safety during the early phase after LDLT has been recently confirmed. In our previous study of 43 sequential liver transplant recipients, we proved its safety without any hepatic arterial complications as well as its beneficial role in recipients with renal dysfunction prior to liver transplantation.18 In this study, we also assessed the proposed role of EVR in reducing HCC recurrence. Although few studies have compared the long-term survival in liver transplant recipients with HCC who received sirolimus and those who received sirolimus-free immunosuppression, such studies on EVR are limited to a few institutional reports.
In this retrospective and prospective analysis, which is a continuation of our previous study,18 we aim to determine the possible impact of EVR in reducing the recurrence of HCC within the UCSF criteria when used in the early phase after LDLT along with Tacrolimus (TAC) based primary immunosuppression.
From January 2011 to June 2013, a total of 250 patients underwent LDLT at the institute of China Medical University Hospital, Taiwan. The patients with HCC within the UCSF criteria were included in this study and they were divided into two groups depending upon postoperative immunosuppression.
Group A: HCC patients who received EVR+TAC based immunosuppressive regimen (n=37) [continuation of our previous study18].
Group B: HCC patients who received standard TAC based immunosuppressive regimen without EVR (n=29).
Both the groups received basiliximab induction therapy and 2 weeks of steroid therapy in tapering doses. EVR was started in group A recipients as early as the 4th to 21st post-operative day after LDLT. The mean follow up period was 46 months (range, 36 to 60 months).
The retrospective data of past medical conditions and demographics were collected. From the point of study enrollment of the patients, the retrospective as well as the prospective data such as laboratory parameters, liver function profile, and imaging studies were collected at regular intervals till the last follow up as per the institute's protocol. Secondary outcomes studied were rates of acute rejection, renal dysfunction, and other adverse effects. Primary end-points of the study were as follows: Loss to follow up, death either due to disease recurrence or any other cause, and recurrence of HCC (Intrahepatic and/or extra-hepatic) during follow up.
If recurrence of HCC was suspected based on findings from investigations such as magnetic resonance imaging (MRI), ultrasonography (USG), rising alpha-fetoprotein (AFP) levels or other clinical findings, dynamic computed tomography (CT) was performed. Findings suggestive of intra-hepatic recurrence of HCC were as follows: Nodule(s) showing hyperdensity during the arterial phase and hypodensity during the portal venous phase of dynamic CT; and for extra-hepatic metastasis, the necessary imaging tests were performed depending upon the location of the tumor, such as chest CT for pulmonary metastasis; brain CT for brain metastasis etc.
When HCC recurrence was diagnosed, prospective data such as date of recurrence, time from transplantation, mode of recurrence (intrahepatic or extrahepatic), and tumor characteristics (number, location, presence of portal vein thrombus) were recorded.
In this study, the overall survival was calculated till the last follow-up period or the death of the recipient starting from LDLT. Disease free survival was defined as the time period from LDLT to the date of the diagnosis of recurrence. The overall and disease-free survival was then compared between the study cohort and the historical control group.
All patients with HCV-related HCC underwent splenectomy in addition to total hepatectomy as per the institution's protocol. After the recipients were discharged with stable liver function, they were required to follow up once a week for the 1st month, every fortnight for the next two months, and then as per the recipient's biochemical profile. Every three months, the blood level of AFP and an abdominal USG were performed for the 1st year.
The immunosuppressive protocol was the same as described in our previous study.18 EVR was started as early as the 4th to 21st post-operative day after LDLT. The target trough level of EVR was 3 to 5 ng/ml, while the target trough level of TAC was 8–10 ng/ml in group A patients, whereas group B patients received only TAC based immunosuppression with similar target trough levels.
Descriptive statistics were computed for age, model for end-stage liver disease (MELD) score, and laboratory data. These statistics were then examined by independent two sample t-tests. Results were presented as mean values±standard deviation (SD). All tests were two-sided, and a p value <0.05 was considered statistically significant. Cox regression model was used to plot the graph.
Group A comprised 37 recipients whereas Group B had 29 recipients who had HCC within the UCSF criteria as the primary indication for LDLT. The mean age of Group A recipients (Male:Female, 28:9) was 56.6 years, while the mean age of Group B patients (Male:Female, 21:8) was 54. 7 years (range, 31 to 69 years). All recipients were followed up for a mean duration of 46 months (36 months to 60 months) after LDLT (Table 1).
The mean value of Alfa-feto protein levels for group A was 7,598.32±8,924.72 ng/ml (range, 1.40 to 54,000 ng/ml) whereas for group B it was 1,016.61±4,730.79 (range, 1.66-25,976) ng/ml. The average MELD score was 13+7 ranging from 8 to 32. In group A patients, the mean trough level of EVR was 3.47±1.53 ng/ml (range, 1.5–11.2 ng/ml) with a daily dose of 1.00±0.25 mg/day. In groups A and B, the average TAC trough levels were 6.97±3.98 ng/ml (range, 2.50 to 11.28 ng/ml) and 6.93±2.58 ng/ml (range, 2–16.30 ng/ml), respectively (Table 2).
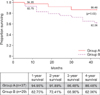
The 1-year, 3-year, and 4-year overall survival rates achieved in Group A patients were 94.95%, 86.48%, and 86.48%, respectively (Table 3), while the 1-year, 3-year, and 4-year overall survival rates achieved in Group B patients were 82.75%, 68.96%, and 62.06%, respectively (p=0.0217). The survival graph is shown in Fig. 1.
Five patients (13.51%) from group A expired. Four patients had extrahepatic recurrence whereas one patient died of sepsis. In group B, eleven patients expired, out of which 7 patients expired due to extrahepatic metastasis of HCC whereas 4 patients died secondary to overwhelming sepsis. All recurrences developed in the extra-hepatic region. None of the studied patients from either of the two groups developed an intra-hepatic recurrence.
Prior renal dysfunction was present in 4 of the recipients (16.66%). Renal function normalized in 2 recipients while it remained stable in 2 recipients. New onset renal dysfunction occurred in 3 recipients. Hyperlipidemia requiring treatment developed in 1 patient.
The leucocyte count was significantly reduced after EVR and tacrolimus combination was used. The mean leucocyte count at the start of EVR was 9.38×103 cells/mm3. In group A recipients, the leucocyte count reduced to 4.95×103 cells/mm3 (p<0.001) at 6 months post-transplantation whereas no such significant drop in the leucocyte count was noticed in group B recipients. Pancytopenia requiring temporary discontinuation of EVR occurred in 1 recipient belonging to group A, while no significant pancytopenia was noticed in group B recipients.
None of the patients developed HAT, incisional hernia, or wound infections, which were the initial concerns. Stomatitis was the most common adverse effect, and it occurred in 14 of the recipients.
Five recipients in this cohort underwent salvage transplantation for recurrent HCC after initial hepatectomy for primary HCC. All of these recipients except for one showed recurrence free survival till the longest available follow up. One recipient from the salvage transplantation subgroup expired at 26 month post transplant. The 1-year, 2-year, and 3-year survival rates in this subgroup were 100%, 100%, and 80%, respectively. None of the studied patients developed acute rejection episodes.
The present prospective and retrospective data of this study cohort shows a positive impact of EVR on reduction of HCC recurrence in recipients who received EVR in the early phase after liver transplantation. The impact was much pronounced in recipients with HCC within the UCSF criteria. With a 4-year cumulative survival of 86.48% in the UCSF subgroup, the role of EVR in reducing HCC recurrences can be positively correlated. Average tumor diameter and major vessel invasion were direct risk factors for recurrence in this study. In the absence of these risk factors, the recurrence of HCC is expected to be even lower.
Prevention and management of HCC recurrence after LDLT are great concerns as the immunosuppressed state after transplantation itself is a causative factor for recurrence of HCC as well as de novo malignancies in recipients. The pro-oncogenic effects of CNIs in causation of de novo malignancies have already been documented in several retrospective and prospective studies.19 Also, studies have shown the correlation between high levels of CNIs in the early transplant period and early HCC recurrence.20 Thus, immunosuppression needs to be tailored carefully in individuals with HCC and HCV infection to prevent recurrence while at the same time avoiding under-immunosuppression that can increase the number of acute rejection episodes. Recently, researchers have described the possible benefits of using mTOR inhibitors in reducing the recurrence of HCC due to their antitumor activities. mTOR signaling plays a role in tumor angiogenesis and proliferation, which is important in carcinogenesis of HCC.21 Hence, reduction in the exposure to CNIs and addition of an mTOR inhibitor as an immunosuppressive agent in the immunosuppressive regimen in the early transplant period can theoretically reduce the risk of HCC recurrence.
After the initial use of mTOR inhibitors, especially sirolimus, in liver transplantation, the enthusiasm waned as the FDA issued a black box warning for de novo sirolimus use in liver transplantation after two preliminary reports showed an increased risk of graft loss and hepatic artery thrombosis (HAT) following its use in liver transplant recipients in the early stage.22 But many studies, predominantly single center and retrospective in design, were published which attempted to refute this association with admittedly modest impact on the mTOR-I use in LT. A recent study by Liang et al.23 not only proved the safety of sirolimus use in a liver transplant population, but it also concluded about its role in reducing HCC recurrence. In their study, sirolimus based regimens decreased tumor recurrence (OR=0.42, 95% CI=0.21–0.83) in comparison with sirolimus-free regimens. However, sirolimus use has been associated with disturbances in hematologic function, including anemia, leukopenia, and thrombocytopenia, which makes sirolimus a less frequently used m-TOR inhibitor.
EVR is a semisynthetic derivative of sirolimus and is well tolerated. Its immunosuppression enhancing role when used in combination with CNIs has been well established in single and multicenter studies that concluded about the safety of EVR use in liver transplant recipients.242526 These studies mainly focused on the superior performance of maintenance EVR (trough levels 3–8 ng/ml) and reduced TAC arm in terms of renal function and biopsy-proven rejection episodes at 12 months after liver transplantation, and justified the use of combination of EVR along with reduced TAC when initiated 30 days or more after transplantation. But, in our previous study, we administered EVR along with TAC as early as the 4th post-operative day after LDLT, and we proved the safety and efficacy of EVR even in the early stage after LDLT. We did not observe any graft-related adverse effects. None of our patients in the previous study suffered from wound infection, hernia, or HAT causing graft loss.18 Thus, considering the safety of EVR in LDLT recipients and its possible anti-tumor role, CNIs can be safely reduced in the early post-operative period with application of EVR in the immunosuppressive regimen.
Clinical trials that have shown the potential role of EVR in reducing the recurrence of HCC after liver transplantation have still not been published. These reports are mainly limited to a few single center, prospective or retrospective studies. Ferreiro et al.10 compared long-term survival and cumulative recurrence in high-risk patients receiving everolimus-based immunosuppression after liver transplantation for HCC with those in an historic control group. In their study, the 5-year cumulative recurrence rate was 61.3% in the control group and 41.3% in the everolimus group. Treatment with everolimus was identified as an independent predictor of longer survival (hazard ratio=0.34; p=.02). In the present study, at the median follow up of 46 months (range, 36–60 months), the recurrence rate was only 10.81% in the EVR group (4/37) whereas one patient in this group died of sepsis. In group B, the recurrence rate was 24.14% (7/29). Four patients from this group expired secondary to sepsis. All three recurrences in this study group showed some specific characteristics: First, all of them showed an extra-hepatic location. Second, all recurrences occurred within 2 years after transplantation. Third, all of the patients who received EVR showed significant leucopenia at 1 year post-transplantation. The pattern of recurrence was particularly important in this study. The possible explanation can be that high immunosuppression in the immediate post-transplantation period can cause proliferation of the disseminated cancer cells that would be uninhibited due to decreased immunity. Hence, introduction of an immunosuppressant that also has anti-tumor activity along with reduction in the number of rejection reactions in the graft can certainly have a positive impact on reducing the recurrence. Although in this study, the average dose of EVR was 1.00±0.25 mg/day with a trough level of 3.47±1.53 ng/ml (range, 1.5–11.2 ng/ml), a larger dose with a higher trough level can certainly increase the anti-tumor effects of EVR if the safety margin of the drug is maintained.
In the present study population, five patients underwent salvage transplantation. The 3-year survival was 80% in this subgroup (4 of 5). Whether EVR has a direct impact on survival after salvage transplantation remains to be proven. But, this finding suggests an improved overall survival of these patients, who had intra-hepatic recurrence after initial liver resection followed by LDLT. However, a long-term follow up of this subgroup is required to prove the role of EVR in reducing the recurrence.
The aim of our previous study18 was to prove the safety of EVR when used in the early phase after LDLT and its efficacy in improving renal function or preventing further deterioration. This study was a continuation of our initial observation in the HCC subgroup, and hence, the dose of EVR was as per the initial protocol. But, with a 4-year survival of 86.48% in the UCSF group, the role and space of this observation can further be expanded by conducting trials of immunosuppressive regimens containing an increased EVR dose with minimal CNI exposure.
In conclusion, EVR use in liver transplant recipients in the early stage after transplantation reduces the HCC recurrence rates among HCC patients within the UCSF criteria. More randomized controlled trials in this regard with higher target trough levels of EVR are warranted to strengthen this finding. The use of EVR was safe without any evidence of HAT or wound infection.
Figures and Tables
References
1. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996; 334:693–699.
2. Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001; 33:1394–1403.
3. Belghiti J, Durand F. Criteria for liver transplantation for hepatocellular carcinoma: what is an acceptable outcome? Liver Int. 2011; 31:Suppl 1. 161–163.
4. Jeng LB, Thorat A, Yang HR, Yeh CC, Chen TH, Hsu CH, et al. Successful use of hepatitis B surface antigen-positive liver grafts - an effective source for donor organs in endemic areas: a single-center experience. Ann Transplant. 2015; 20:103–111.
5. Marsh JW, Dvorchik I. Liver organ allocation for hepatocellular carcinoma: are we sure? Liver Transpl. 2003; 9:693–696.
6. Toso C, Trotter J, Wei A, Bigam DL, Shah S, Lancaster J, et al. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2008; 14:1107–1115.
7. Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009; 10:35–43.
8. Chan SC, Fan ST, Chok KS, Cheung TT, Chan AC, Fung JY, et al. Survival advantage of primary liver transplantation for hepatocellular carcinoma within the up-to-7 criteria with microvascular invasion. Hepatol Int. 2011; 6:646–656.
9. Lei JY, Wang WT, Yan LN. Up-to-seven criteria for hepatocellular carcinoma liver transplantation: a single center analysis. World J Gastroenterol. 2013; 19:6077–6083.
10. Ferreiro AO, Vazquez-Millán MA, López FS, Gutiérrez MG, Diaz SP, Patiño MJ. Everolimus-based immunosuppression in patients with hepatocellular carcinoma at high risk of recurrence after liver transplantation: a case series. Transplant Proc. 2014; 46:3496–3501.
11. Zimmerman MA, Trotter JF, Wachs M, Bak T, Campsen J, Skibba A, et al. Sirolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Liver Transpl. 2008; 14:633–638.
12. Penn I, Starzl TE. Malignant tumors arising de novo in immunosuppressed organ transplant recipients. Transplantation. 1972; 14:407–417.
13. Aberg F, Pukkala E, Höckerstedt K, Sankila R, Isoniemi H. Risk of malignant neoplasms after liver transplantation: a population-based study. Liver Transpl. 2008; 14:1428–1436.
14. Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999; 397:530–534.
15. Maluccio M, Sharma V, Lagman M, Vyas S, Yang H, Li B, et al. Tacrolimus enhances transforming growth factor-beta1 expression and promotes tumor progression. Transplantation. 2003; 76:597–602.
16. Vivarelli M, Cucchetti A, Piscaglia F, La Barba G, Bolondi L, Cavallari A, et al. Analysis of risk factors for tumor recurrence after liver transplantation for hepatocellular carcinoma: key role of immunosuppression. Liver Transpl. 2005; 11:497–503.
17. Schumacher G, Oidtmann M, Rosewicz S, Langrehr J, Jonas S, Mueller AR, et al. Sirolimus inhibits growth of human hepatoma cells in contrast to tacrolimus which promotes cell growth. Transplant Proc. 2002; 34:1392–1393.
18. Jeng LB, Thorat A, Hsieh YW, Yang HR, Yeh CC, Chen TH, et al. Experience of using everolimus in the early stage of living donor liver transplantation. Transplant Proc. 2014; 46:744–748.
19. Moini M, Schilsky ML, Tichy EM. Review on immunosuppression in liver transplantation. World J Hepatol. 2015; 7:1355–1368.
20. Rodríguez-Perálvarez M, Tsochatzis E, Naveas MC, Pieri G, García-Caparrós C, O'Beirne J, et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J Hepatol. 2013; 59:1193–1199.
21. Finn RS. Current and future treatment strategies for patients with advanced hepatocellular carcinoma: Role of mTOR inhibition. Liver Cancer. 2012; 1:247–256.
22. Wiesner R, Klintmalm G, McDiarmid S;. Sirolimus immunotherapy results in reduced rates of acute rejection in de novo orthotopic liver transplant recipients. Am J Transplant. 2002; 2:464. Abstract 1294.
23. Liang W, Wang D, Ling X, Kao AA, Kong Y, Shang Y, et al. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2012; 18:62–69.
24. Saliba F, Dharancy S, Lorho R, Conti F, Radenne S, Neau-Cransac M, et al. Conversion to everolimus in maintenance liver transplant patients: a multicenter, retrospective analysis. Liver Transpl. 2011; 17:905–913.
25. Fischer L, Klempnauer J, Beckebaum S, Metselaar HJ, Neuhaus P, Schemmer P, et al. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation--PROTECT. Am J Transplant. 2012; 12:1855–1865.
26. Levy G, Schmidli H, Punch J, Tuttle-Newhall E, Mayer D, Neuhaus P, et al. Safety, tolerability, and efficacy of everolimus in de novo liver transplant recipients: 12- and 36-month results. Liver Transpl. 2006; 12:1640–1648.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download