Abstract
Backgrounds/Aims
To achieve complete anatomic hepatectomy in a large hepatocellular carcinoma (HCC), hepatic transection through an anterior approach is required. Liver hanging maneuver (LHM) is a useful procedure for transection of an adequately cut plane in anatomical liver resection. It may reduce intraoperative bleeding and transection time.
Methods
We examined records of 27 patients with large HCC (over 10 cm in size) who underwent anatomic hepatic resection with LHM (n=11, between 2001 and 2007) or without LHM (n=16, between 2000 and 2003). The two groups were retrospectively compared in terms of patient demographics, preoperative hepatic function, surgical records, and post-hepatectomy outcome.
Results
Although transection time was not significantly different between the two groups, the amount of intraoperative blood loss was significantly lower in the LHM group than that in the non-LHM group (1,269±1,407 ml vs. 2,197±1,281 ml, p=0.039). Related blood transfusion or total operation time in the LHM group tended to be lower than those in the non-LHM group, although differences between the two groups were not statistically significant (p<1.0). Prevalence of total complications in the LHM group tended to be lower than that in the LHM group (36% vs. 88%, p=0.011). However, prevalence of hepatectomy-related complications or length of hospital stay was not significantly different between the two groups.
Anatomic hepatic resection refers to the removal of a liver segment confined by Glissonian branches. Theoretically it is a logical procedure that can eradicate intrahepatic metastasis of hepatocellular carcinoma (HCC), minimize postoperative tumor relapse, and prolong survival of HCC patients.12 To achieve complete anatomic resection in major hepatectomy, it is necessary to cut adequate portal pedicles to ensure sufficient exposure of hepatic veins. In such liver resection, transecting the liver parenchyma through an anterior approach without mobilizing the remnant liver is preferred because avoiding liver rotation has advantages of circumventing tumor dissemination and/or injury produced by compression of the remnant liver.34 However, it is sometimes difficult to achieve appropriate transection, particularly when liver tumor is large. A longer transection time may also increase blood loss.
Belghiti et al.5 have proposed liver hanging maneuver (LHM) for right hepatectomy without liver mobilization using a tape inserted between the anterior surface of the vena cava and the liver. Lifting this tape allows easier parenchymal transection in the deeper site with good control of bleeding. Since its introduction by Belghiti et al.,5 LHM has gained worldwide popularity for major hepatectomy or hepatectomy, particularly for large-sized liver tumor or tumor invading surrounding tissues.5678 Recently, this technique has been applied for various anatomical resections.910 According to recent development of hepatic transection, the deeper part seemed to be lifted. In a large-size HCC, the trunk of the Glissonian pedicle, including the intrahepatic vasculature and hepatic veins, is often severely compressed. Therefore, adequate transection plane may not be obtained during transection. Both transected and the remnant liver can be rotated toward the counterside by using LHM in the case of a large liver tumor during transection,11 making it easier to transect the deeper parenchyma in the final step successfully. Thus, adequate transection might be achieved by using LHM even in such a large HCC. We hypothesized that LHM could provide an adequately cut plane, leading to minimal blood loss and/or reduced transection time.
To test our hypothesis, we retrospectively and historically examined surgical data in large-sized (>10 cm) HCC of patients who underwent major hepatectomy and compared the parameters with those of patients who underwent liver resection with or without LHM.
The study protocol for database access and review, ethics, and non-conflict of interests was approved by the Institutional Medical Board of Nagasaki University Hospital on April 17, 2017 (approval number: 17041705). A signed consent from each patient for studying their clinical data was obtained before surgery. Patient agreement was obtained by an opt-out procedure performed by a co-author (Y.S.).
Between 1997 and March 2015, we performed 27 liver resections (more than 3-segmentectomies) in patients with large HCC (over 10 cm in size) among 621 liver resections. Since November 2001, we began to apply the method described by Belghiti et al.5 for LHM. A total of 11 patients who underwent resections by applying LHM since 2001 (the LHM group) were compared to a total of 16 patients who underwent resections without LHM between 1997 and 2003 (the non-LHM group). The surgical team was fixed during this period. Three experienced surgeons performed all hepatectomies. The following data were collected for analysis: age, gender, background liver disease, liver disease, preoperative liver functions (indocyanine green retention rate at 15 minutes [ICGR15]), liver uptake ratio by technetium-99m-galactosyl, human serum albumin, liver scintigraphy (LHL15), liver damage grade guided by the General Rules for the Clinical and Pathological Study of Primary Liver Cancer in Japan,12 surgical procedure or records (presence of thoracotomy, extent of hepatectomy, surgical device, presence of vena cava clamping, operation time, time of liver parenchymal transection, blood loss, blood transfusion), postoperative complications (including uncontrolled ascites, significant bile leak, and intraabdominal infections), and duration of hospitalization after the operation. Bile leakage was defined as a bile discharge at the cut surface of the liver. Long-term ascites or pleural effusion was defined as intra-abdominal or thoracic fluid collection while under the use of diuretics over two weeks. Intraabdominal infection was defined as the presence of intraabdominal fluid with obvious infectious discharge or with septic systemic findings. Hepatic failure and hospital-stay death were not observed in this series.
Of 11 hepatectomies performed with LHM, nine were hemi-hepatectomies, one was extended hemi-hepatectomy, and one was right trisectionectomy. Of 16 non-LHM hepatectomies, eight were hemi-hepatectomies, seven were extended hemi-hepatectomies, and one was right trisectionectomy.
The surgical procedure included a J-shaped incision laparotomy (upper median plus right-sided transverse incision to the ninth intercostal space) which was performed in a basic manner.13 The falciform ligament was cut to expose confluences of right, middle, and left hepatic veins as well as the anterior surface of the vena cava. Mobilization of the remnant liver was not carried out in patients who underwent LHM. LHM was basically conducted according to the method described by Belghiti et al.5 Briefly, the space between the right and middle hepatic veins (RHV and MHV, respectively) was dissected using a right-angled clamp. From this space, loose connective tissues between the anterior surface of the vena cava and the paracaval caudate lobe were dissected over a 3-cm length using a long right-angled clamp because short hepatic veins were absent in this space.14 Subsequently, the space between the vena cava and infra-hepatic caudate process was dissected and a few short hepatic veins were divided. The loose tissue in this space was dissected using a long, light, and curved Kelly clamp. A 10-Fr size nasogastric (NG) tube was inserted between the RHV and MHV. It was then passed easily through the dissecting space.15 We completed tube insertion within approximately 20 min. After the insertion, the tube was lifted for LHM. The cut plane along the middle or umbilical fissure hepatic vein was then hung up by the tube (Fig. 1). For right trisectionectomy, the tube was repositioned between the confluence of the middle and left hepatic veins. It was then placed adjacent to the umbilical Glisson's pedicle at the hepatic hilum. As previously described, various anatomical resections are possible by tube re-positioning technique as described by Kokudo et al.16 The transection method was similar between the two patient groups during the study period. Coagulation instruments were not used in our institute before 2008. Since 2008, hemostatic devices such as Ligasure® and ultrasonic coagulation instruments have been used in both groups.17 Hepatic transection was performed mainly combined with the crush clamping method. An ultrasonic dissector was used for dissection around main vessels at the hepatic hilum or inferior vena cava.17 The hepatic inflow was intermittently occluded during transection using Pringle maneuver (15-minute occlusion and 5-minute de-clamping).18 The LHM tube was always pulled up during transection. The direction of the transection was always targeted toward the hanging tube. By maintaining the position of the tube, transection to the anterior aspect of the vena cava could be performed easily. When bleeding of the compressed hepatic vein could not be controlled during hepatectomy, the infra-hepatic vena cava was taped and a semi-clamp was performed by maintaining central venous pressure.19
Continuous data are expressed as mean±SD. Data for different groups were compared using one-way analysis of variance (ANOVA). Chi-square test was used to compare categorical variables. Differences between groups were analyzed by Fisher's exact test or Scheffé's multiple comparison test. A two-tailed p-value of less than 0.05 was considered statistically significant. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) software, version 20.0 (IBM, Chicago, IL, USA).
Demographic and surgical data with comparison between the LHM and non-LHM hemihepatectomy groups are summarized in Table 1. Patients of the two groups had similar age and gender. Comparisons for background liver diseases and liver tumors showed no significant differences between the two groups. Preoperative liver function tests were not significantly different between the two groups either.
Operative procedure, presence of thoracotomy, extent of hepatectomy, and the use of vena cava clamping and surgical devices were not significantly different between the two groups. Although transection time (almost equal to the time of clamping of hepatic blood inflow) was not significantly different between the two groups, the amount of intraoperative blood loss in the LHM group was significantly (p<0.05) lower than that in the non-LHM group. Related blood transfusion time and total operation time in the LHM group tended to be lower than those in the non-LHM group, although differences between the two groups were not statistically significant (p<0.10).
Comparison results for postoperative complications and outcomes between the two groups are summarized in Table 1. Regarding postoperative complications, the prevalence of total complications in the non-LHM group tended to be lower than that in the LHM group (p<0.01). However, the prevalence of hepatectomy-related complications was not significantly different between the two groups. The length of hospital stay was not significantly different between the two groups either. No hospital death was recorded in the present series.
Since the report of LHM by Belghiti et al.,5 it has been widely applied in liver resection.5678910112021222324 In hepatic malignancy, mobilization or rotation of the resected liver with a liver tumor should be avoided to prevent tumor dissemination during operation.23 In patients with a large tumor or a tumor that invades adjacent organs, anterior liver transection through the LHM procedure is preferred. Hepatic vein transection or combined resection of invasive parts can be done in the final step after complete transection to the front of the vena cava. Once the tape or tube is placed at the retrohepatic space on the vena cava, transection in the deeper part or near the trunk of the hepatic veins can be safely performed because the lifting tape is always detected as a marker of an adequate transection plane. Thus, injury to tiny branches of the hepatic vein or short hepatic vein can be reduced. Kokudo et al.16 have proposed a gradual tape-repositioning technique in cases of living liver donation. In this technique, the tape is inserted by passing it between Glisson's pedicle and liver parenchyma. We prefer to use this re-positioning technique of LHM in right trisectionectomy or left hemihepatectomy by placing the tape between the middle and left hepatic veins. Through this technique, LHM can be applied in various anatomical resections.91025 We have already published a case report as a preliminary trial of LHM in trisectionectomy for a huge liver tumor.26 In this study, we performed retrospective analysis for 11 patients with large-sized HCC who underwent hepatic resection with LHM.
In the present study, we compared clinical parameters and outcomes between LHM and non-LHM groups for large HCC in a non-random fashion. The decision to use LHM actually depended on the first author during the study period. As shown in Table 1, background or preoperative liver functional reserve of HCC patients in both groups was not significantly different in our series, although the period of using LHM was different. Surgical records showed that LHM was used to reduce blood loss in particular, as stated in a previous report.11 By lifting the tape during transection, bleeding from the hepatic vein branches or the trunk itself by compressing the huge HCC might be adequately controlled. This might be due to the following reasons. First, dissecting the liver parenchyma was more rapidly performed by parenchymal compression using LHM. Second, transecting in the deeper part near the vena cava might be limited without worrying about injury to cava or short hepatic veins by the shield of the covering tape. Third, the operator could always target the tape position during transection. Therefore, so an adequate transection plane could be obtained without hesitation. Clamping of the vena cava19 may prevent bleeding of the hepatic veins due to decreased central venous pressure. However, most cases did not receive this useful procedure. We now apply caval semi-clamp in case the cut plane is set along hepatic veins or the tumor located adjacent to the hepatic vein. Therefore, related blood transfusion tended to be reduced. This was decided by the anesthesiologist in this series. In the present study, the significantly decreased time required for hepatic parenchymal transection in the LHM group is currently unclear. The operating time tended to be shorter in the LHM group than that in the non-LHM group, similar to reports of previous studies.2728 Although additional time was required to prepare for LHM, our analysis showed that total operating time was actually saved. It has been reported that the required time for dissection around resected liver can be saved by an anterior approach using LHM.29 Difference in transecting devices might not influence surgical records because of their powerful shielding ability.17
Our results showed that the prevalence of postoperative morbidity such as ascites, bile leakage, or intraabdominal infection was not significantly different between the two groups, although the prevalence of total postoperative complications was reduced in the LHM group in comparison with that in the non-LHM group in this study. Our previous study has shown that postoperative deterioration of liver functions is avoided in the LHM group and that the prevalence of uncontrolled ascites is limited in total hepatectomy applying LHM.27 By selecting a large HCC, LHM might not lead to improvement of postoperative course. Ascites might be produced by the dissection of surrounding hepatic ligaments for mobilization or following lymph node dissection. Regarding node dissection, this technique was not necessary for the two groups. Most patients in this series actually did not receive node dissection. Limited liver mobilization by applying LHM might have avoided respiratory and cardiac complications. On the other hand, long-term ascites noted in some patients after operation with injured liver might have contributed to longer hospitalization time (no significance data). The development of ascites might have influenced the duration of hospitalization. When intra-operative rotation of transected liver was limited, contamination of bile or other pathogens to the abdominal wall that leads to superficial surgical site infections might have been avoided in this series.
We believe that the most important advantage of LHM is that it helps confirm the appropriately cut plane during transection in large HCC or other expanding liver tumors.27 Because the tape is placed adjacent to the hepatic vein, appropriate transection could be accomplished by LHM when the first cut-line is appropriately obtained. Due to the limited number of hepatectomy for large HCC (over 10 cm) in the present study, we cannot make firm conclusions regarding the usefulness of LHM based on present results of postoperative outcomes at this stage. However, LHM seems to be useful for hepatic transection along the hepatic vein even in case of compression by a large HCC. Further study using a larger number of patients would be necessary to clarify the utility of LHM.
In summary, we examined the suitability of LHM for major hepatectomy in large-sized HCC through a retrospective cohort study. The use of LHM reduced intraoperative blood loss and tended to decrease red cell transfusion or shorten the operating time. LHM can be applied to transection at adequate cut plane in large-sized HCC. However, the prevalence of hepatectomy-related complications or hospital stay was not improved by LHM. Further study is needed to clarify the utility of LHM.
References
1. Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, et al. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005; 242:252–259. PMID: 16041216.

2. Emond JC, Polastri R. Anatomical hepatectomy for resection or transplantation. Am J Surg. 1996; 172:29–34. PMID: 8686798.

3. Liu CL, Fan ST, Cheung ST, Lo CM, Ng IO, Wong J. Anterior approach versus conventional approach right hepatic resection for large hepatocellular carcinoma: a prospective randomized controlled study. Ann Surg. 2006; 244:194–203. PMID: 16858181.
4. Yamanaka N, Okamoto E, Fujihara S, Kato T, Fujimoto J, Oriyama T, et al. Do the tumor cells of hepatocellular carcinomas dislodge into the portal venous stream during hepatic resection? Cancer. 1992; 70:2263–2267. PMID: 1327495.

5. Belghiti J, Guevara OA, Noun R, Saldinger PF, Kianmanesh R. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg. 2001; 193:109–111. PMID: 11442247.

6. Hirai I, Murakami G, Kimura W, Kanamura T, Sato I. How should we treat short hepatic veins and paracaval branches in anterior hepatectomy using the hanging maneuver without mobilization of the liver? An anatomical and experimental study. Clin Anat. 2003; 16:224–232. PMID: 12673817.
7. Suzuki M, Unno M, Katayose Y, Takeuchi H, Rikiyama T, Onogawa T, et al. Hepatic resection through an anterior approach employing a modified liver hanging maneuver in patients with a massive liver tumor severely oppressing the inferior vena cava. Hepatogastroenterology. 2004; 51:1459–1463. PMID: 15362776.
8. Ogata S, Belghiti J, Varma D, Sommacale D, Maeda A, Dondero F, et al. Two hundred liver hanging maneuvers for major hepatectomy: a single-center experience. Ann Surg. 2007; 245:31–35. PMID: 17197962.
9. Kim SH, Park SJ, Lee SA, Lee WJ, Park JW, Hong EK, et al. Various liver resections using hanging maneuver by three Glisson's pedicles and three hepatic veins. Ann Surg. 2007; 245:201–205. PMID: 17245172.

10. Nanashima A, Nagayasu T. Development and clinical usefulness of the liver hanging maneuver in various anatomical hepatectomy procedures. Surg Today. 2016; 46:398–404. PMID: 25877717.

11. Wang CC, Jawade K, Yap AQ, Concejero AM, Lin CY, Chen CL. Resection of large hepatocellular carcinoma using the combination of liver hanging maneuver and anterior approach. World J Surg. 2010; 34:1874–1878. PMID: 20414779.

12. Japanese Society for Cancer of the Colon and Rectum. The general rules for clinical and pathological studies on cancer of the colon, rectum and anus (in Japanese). 7th ed. Tokyo: Kanehara & Co., Ltd;2006. p. 14–15.
13. Makuuchi M, Yamamoto J, Takayama T, Kosuge T, Gunvén P, Yamazaki S, et al. Extrahepatic division of the right hepatic vein in hepatectomy. Hepatogastroenterology. 1991; 38:176–179. PMID: 1649789.
14. Sato TJ, Hirai I, Murakami G, Kanamura T, Hata F, Hirata K. An anatomical study of short hepatic veins, with special reference to delineation of the caudate lobe for hanging maneuver of the liver without the usual mobilization. J Hepatobiliary Pancreat Surg. 2002; 9:55–60. PMID: 12021898.

15. Nanashima A, Tobinaga S, Abo T, Sawai T, Nagayasu T. Left hepatectomy accompanied by a resection of the whole caudate lobe using the dorsally fixed liver-hanging maneuver. Surg Today. 2011; 41:453–458. PMID: 21365437.

16. Kokudo N, Sugawara Y, Imamura H, Sano K, Makuuchi M. Sling suspension of the liver in donor operation: a gradual tape-repositioning technique. Transplantation. 2003; 76:803–807. PMID: 14501857.

17. Nanashima A, Abo T, Arai J, Takagi K, Matsumoto H, Takeshita H, et al. Usefulness of vessel-sealing devices combined with crush clamping method for hepatectomy: a retrospective cohort study. Int J Surg. 2013; 11:891–897. PMID: 23954369.

18. Man K, Fan ST, Ng IO, Lo CM, Liu CL, Yu WC, et al. Tolerance of the liver to intermittent pringle maneuver in hepatectomy for liver tumors. Arch Surg. 1999; 134:533–539. PMID: 10323426.

19. Rahbari NN, Zimmermann JB, Koch M, Bruckner T, Schmidt T, Elbers H, et al. IVC CLAMP: infrahepatic inferior vena cava clamping during hepatectomy--a randomised controlled trial in an interdisciplinary setting. Trials. 2009; 10:94. PMID: 19825186.

20. Suh KS, Lee HJ, Kim SH, Kim SB, Lee KU. Hanging maneuver in left hepatectomy. Hepatogastroenterology. 2004; 51:1464–1466. PMID: 15362777.
21. Kim SH, Park SJ, Lee SA, Lee WJ, Park JW, Kim CM. Isolated caudate lobectomy using the hanging maneuver. Surgery. 2006; 139:847–850. PMID: 16782444.

22. Ettorre GM, Vennarecci G, Santoro R, Antonini M, Lonardo MT, Carlini M, et al. Modified liver hanging maneuver during orthotopic liver transplantation with inferior vena cava preservation. Transplantation. 2003; 75:247–249. PMID: 12548135.

23. Sheen IS, Jeng KS, Shih SC, Wang PC, Chang WH, Wang HY, et al. Does surgical resection of hepatocellular carcinoma accelerate cancer dissemination? World J Gastroenterol. 2004; 10:31–36. PMID: 14695764.

24. Gaujoux S, Douard R, Ettorre GM, Delmas V, Chevallier JM, Cugnenc PH. Liver hanging maneuver: an anatomic and clinical review. Am J Surg. 2007; 193:488–492. PMID: 17368296.

25. Liddo G, Buc E, Nagarajan G, Hidaka M, Dokmak S, Belghiti J. The liver hanging manoeuvre. HPB (Oxford). 2009; 11:296–305. PMID: 19718356.

26. Nanashima A, Sumida Y, Abo T, Takeshita H, Hidaka S, Sawai T, et al. Trisectionectomy for large hepatocellular carcinoma using the liver hanging maneuver. Eur J Surg Oncol. 2009; 35:326–330. PMID: 18316172.

27. Nanashima A, Sumida Y, Abo T, Nagayasu T, Sawai T. Usefulness and application of the liver hanging maneuver for anatomical liver resections. World J Surg. 2008; 32:2070–2076. PMID: 18452024.

28. Beppu T, Ishiko T, Chikamoto A, Komori H, Masuda T, Hayashi H, et al. Liver hanging maneuver decreases blood loss and operative time in a right-side hepatectomy. Hepatogastroenterology. 2012; 59:542–545. PMID: 22353521.
29. Capussotti L, Ferrero A, Russolillo N, Langella S, Lo Tesoriere R, Viganò L. Routine anterior approach during right hepatectomy: results of a prospective randomised controlled trial. J Gastrointest Surg. 2012; 16:1324–1332. PMID: 22570073.

Fig. 1
A large HCC compressing the cut-plane along the umbilical fissure vein for right trisectionectomy. The hanging tube is a marker for the first cutting place.
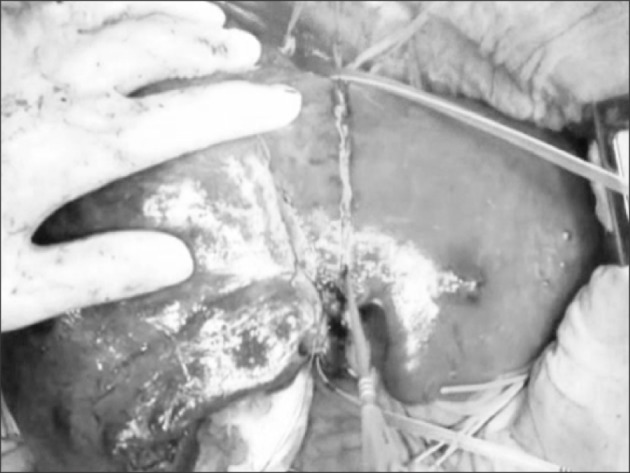
Table 1
Comparison of patient demographics, surgical record, and postoperative outcomes
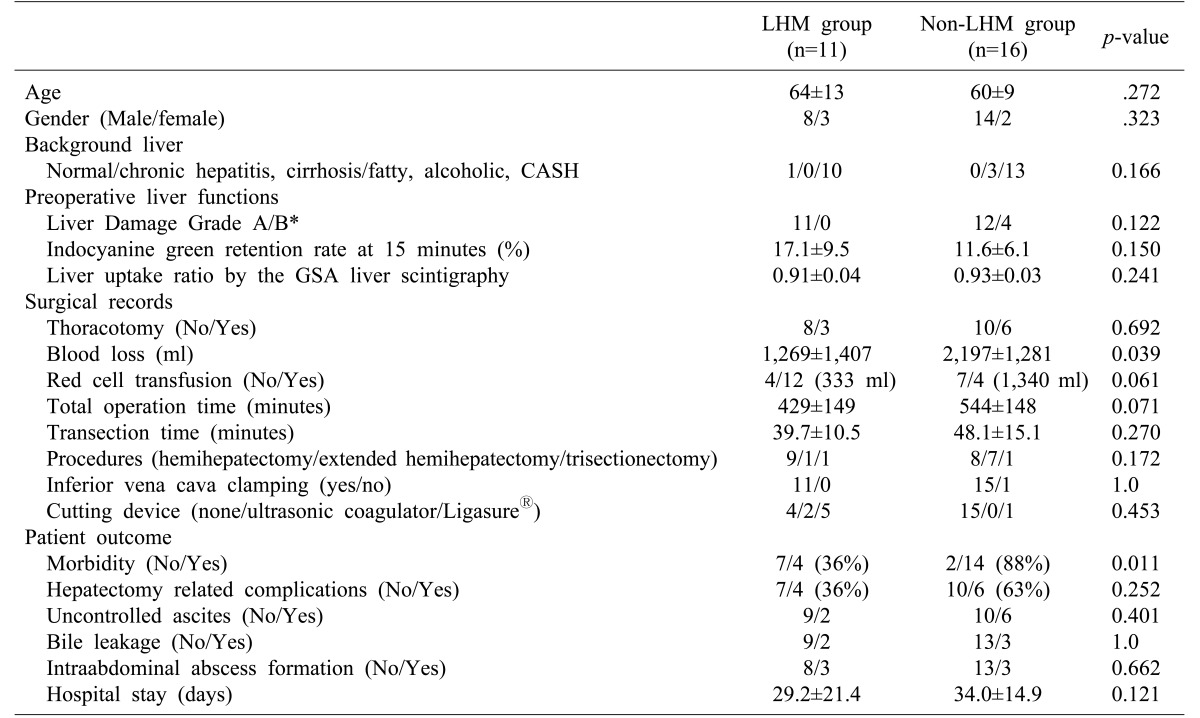
*Liver Damage grade guided by the General Rules for the Clinical and Pathological Study of Primary Liver Cancer in Japan.12
GSA: galactosyl serum albumin




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download