Abstract
Peliosis Hepatis (PH) is a rare vascular disorder of the liver, characterized by the presence of cystic blood-filled cavities distributed throughout the hepatic parenchyma. The pathogenesis of PH remains controversial. The preoperative diagnosis of PH is difficult, due to the non-specific imaging characteristics of PH and almost all cases are diagnosed on histology post resection. This study presents a case of PH masquerading as hepatocellular carcinoma (HCC). The patient is a 45-year old Chinese lady, who presented with transaminitis. She was found to be hepatitis B virus core total antibody-positive with an alpha-fetoprotein (AFP) of 29.4 ng/ml. Triphasic liver computed tomography showed several arterial hypervascular lesions and hypoenhancing lesions on the venous phase, particularly in the segments 6/7. Subsequently, a magnetic resonance imaging scan showed multiple lesions in the right hemiliver with an indeterminate enhancement patterns. Subsequently, she decided to undergo a resection procedure. Histopathology revealed findings consistent with PH with some unusual features. This case demonstrates a clinical conundrum, in which PH presented with a raised AFP, in a patient with risk factors for the development of HCC. The clinical suspicion of PH should be high in patients, who present with multiple hepatic lesions with variable enhancement patterns.
Peliosis is an uncommon vascular disorder with an unknown pathogenesis. It is characterized by blood-filled cysts within parenchymatous organs, of which, the liver is most commonly involved. Peliosis hepatis (PH) is increasingly being recognized as a differential diagnosis for hepatic lesions. However, given non-specific imaging findings and enhancement patterns, histopathology is often required for a definitive diagnosis of this entity. In recent years, there have been an increasing number of case reports in the literature, describing PH and its associated causative risk factors. We describe a case of PH masquerading as hepatocellular carcinoma (HCC), in a patient with antibodies to hepatitis B core antigen (anti-HBc total) and raised alpha-fetoprotein (AFP) levels.
Institutional Review Board approval was not required for this paper as it describes only a single subject.
This report presents a case of a 45 year old Chinese female, who was referred to the National University Hospital in Singapore for further investigation of her transaminitis. Further investigations revealed she had raised AFP levels (29.4 ng/ml) and was anti-HBc Total positive. Laboratory results at the patient's first visit are presented in Table 1.
The patient had a medical history of multinodular goiter, for which she underwent a thyroid nodule excision 20 years prior and subsequently, a left hemithyroidectomy in 2006. Both procedures reported benign pathology. The patient had a termination of pregnancy in 2013, for which she was given a prostaglandin analogue. In 2014, the patient was seen by a gynecologist for investigation of menorrhagia, for which she underwent a hysteroscopy. Histology revealed benign mixed proliferative and secretory endometrium.
The patient did not take any oral contraceptives nor did she consume other regular medications such as steroids, hormonal medications, antibiotics, or any traditional medications. She had no past history of infections such as Human Immunodeficiency Virus (HIV) or Tuberculosis and reported no symptoms of fever, loss of weight, appetite or night sweats. She had no significant family history of malignancy or chronic infections and had no history of smoking or regular alcohol intake. Physical examination was unremarkable.
She was further investigated with a triphasic liver computed tomography (CT) scan, which reported 3 arterial enhancing foci, which were not visible in the venous and delayed phases – in segment 6, 3, and 2. In the portal venous phase, there were heterogeneous hypodensities in the segments 6 (which were enhancing on the arterial phase), 7, and 8. CT images are shown in Fig. 1. Given the indeterminate nature of a number of lesions, she subsequently underwent a magnetic resonance imaging (MRI) scan of the liver with Primovist, which revealed multiple T2 isointense to hyperintense and T1 isointense to hypointense lesions scattered throughout both lobes – the largest at Segment 6/7, measuring up to 1.9 cm. The larger lesions were seen to enhance predominantly in the portal venous phase. Many of them showed an irregular pattern of enhancement. In the delayed phase, the lesions became increasingly isointense, and with 20 minutes of delay, the larger lesions were slightly hypointense. MRI images are shown in Fig. 2.
After a discussion at the hospital's Multidisciplinary Tumor Board Meeting, the patient was offered a percutaneous biopsy or hepatic resection. Given the non-specific findings, the possibility of a benign pathology was conveyed to the patient. However, the patient opted for surgical resection, in view of the risk of bleeding and other complications from a biopsy and a false negative result.
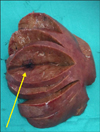
Subsequently, she underwent an open right hepatectomy. Multiple hypodense lesions in the right lobe were seen on Intraoperative Ultrasound (IOUS). There were no peritoneal or hepatic nodules. Pictures of the resected/cut specimen are shown in Fig. 3. She recovered following the procedure.
A histological examination showed a non-cirrhotic liver with multiple, irregular and ill-defined hemorrhagic lesions composed of blood-filled cystically dilated spaces without a definite endothelial lining. Some of the lesions were clustered together and bordered central veins. Unusually, the blood lakes contained an abundant meshwork of reticulin fibers with neoangiogenesis, without accompanying stroma, granulation tissue, or a surrounding fibrous capsule. Warthin-Starry stain was negative, excluding bacillary angiomatosis. Small nascent blood-filled lesions were visible within the space of Disse. The residual liver parenchyma away from the lesions also showed dilated true sinusoidal spaces. No dysplasia or malignancy was observed. These findings were consistent with PH, with some unusual features. Histology slide images are presented in Fig. 4.
This is the first case of PH reported, that has been associated with a raised AFP. There is increasing evidence that patients who are found to be anti-HBc-positive are at an increased risk of developing HCC.1 A recent meta-analysis by Coppola et al.,2 which analysed 26 studies with 44,553 patients, demonstrated that the relative risk of HCC amongst HBsAg negative but anti-HBc-positive subjects was 1.67 times that compared to those who were anti-HBc negative. Complementary to this, Coppola et al.3 showed in a separate study, in which they sought hepatitis B virus (HBV) DNA in HCC and non-HCC hepatic parenchyma, that patients who were anti-HBs negative but anti-HBc positive had a significant risk of having occult HBV infection. They found HBV DNA in 54.5% of patients. It is thus clear that this patient, who was found to be HBsAg negative but anti-HBc positive, had an increased risk of developing HCC. Hence, the approach for this patient with new hepatic lesions seen on imaging, coupled with a raised AFP, demanded an exclusion of HCC.
Given the dual blood supply of the liver, enhancement patterns of hepatic lesions assist greatly in the diagnosis of hepatic lesions. The typical features of HCC, hepatic metastases, hemangiomas, and other hepatic lesions have been described extensively and have high diagnostic accuracy. However, the preoperative diagnosis of PH remains difficult, mainly due to the variability and non-specificity of imaging findings.456 This results in most cases of PH being diagnosed post-resection on histopathological examination. On Computer Tomography (CT) imaging, the characteristics of PH are dependent on the degree of thrombosis and hemorrhage within the lesion, the extent of communication with sinusoids, and the degree of steatosis within the liver parenchyma.7 On unenhanced CT, PH is typically hypo-attenuating compared to the surrounding liver parenchyma.8 On contrast-enhanced CT, PH tends to be hypo-attenuating to the surrounding liver in early acquisitions, but become iso-attenuating with time. Unique features during the arterial phase include globular and target lesions.89 In the venous phase, lesions typically become iso or hyper-attenuating,10 although lesions with active hemorrhage might show little enhancement. The enhancement may be complete or may show early globular enhancement with progressive centrifugal or centripetal contrast uptake on portal venous phases.1011 In the delayed phase, diffuse increased attenuation may be seen.12 The patient was seen to have contrast enhancement patterns atypical for PH, with the lesions showing arterial enhancement and heterogenous hypodensity in the portal venous phase. Furthermore, some lesions, which were arterially enhancing, were isodense and not seen on the portal venous phase. Other lesions which were subsequently seen as hypodense on the portal venous phase were isodense on the arterial phase. On MRI, PH is generally hyperintense, compared to the surrounding liver parenchyma on T2-weight sequences, with reports describing multiple foci of high signal, likely due to areas of hemorrhagic necrosis.8 On T1-weighted series, lesions are typically hypo-intense, due to the presence of subacute blood, but can also appear be iso- or hyper-intense.131415 Lesions commonly show enhancement with contrast administration, mostly with a centrifugal pattern.16 Kim et al.'s series17 of 8 patients with histologically proven PH, in which they correlated pathologic and imaging findings, showed that homogenously high enhancement correlated with dilated sinusoids filled with fresh blood. Five patients showed centripetal or persistently low enhancement pattern and histology showed sinusoids filled with old stagnated blood - further confirmation that variable enhancement patterns are linked to the state and timing of recent hemorrhage. This patient showed more typical findings on MRI, with multiple T2 iso- to hyperintense lesions, and T1 iso to hypointense lesions scattered throughout the liver. It is clear that depending on the individual characteristics of the lesion, PH can mimic a multitude of other neoplastic lesions of the liver. However, the findings of variable enhancement patterns across multiple lesions should raise the suspicion for diagnosis.
Peliosis is an uncommon vascular disorder characterized by blood-filled cysts within parenchymatous organs, where the liver is most commonly involved.18192021 The term Peliosis Hepatis (PH) was coined by Schoenlank in 1916, in which he described the condition in a young lady who had military tuberculosis.18 Since Schoenlank's report, there have been multiple case reports and series describing the clinical, morphological, and histopathological characteristics of PH. PH is most commonly found as an incidental finding, following cross sectional imaging. However, it can present acutely with hepatic rupture.102223 The risk of rupture has been reported to be dependent on the location of the lesions, with 75% of right sided lesions, 11% of left sided lesions, and 14% of bilobar lesions presenting with rupture.24 While the pathogenesis of PH remains controversial, early papers have suggested that it is associated with possible outflow obstruction with subsequent sinusoidal hypertension. This results in hepatocyte necrosis and sinusoidal wall weakness.25 Other proposed mechanisms include: (1) congenital malformation or vascular varicosity, (2) hepatocyte necrosis with cavity formation, (3) reticulin fibre breakdown with sinusoidal dilatation, or (4) the breakdown of sinusoidal borders with increased endothelial cell permeability and infiltration of red blood cells into the space of Disse.4 Zafrani et al.26 studied the ultrastructure in 12 cases of PH and found multiple alterations of the sinusoidal membrane including multiple blebs and subsequent peri-sinusoidal fibrosis. This led them to suggest that alterations in the sinusoidal barrier might constitute the primary triggering event in the development of PH.
In terms of etiology, PH has been associated with medications such as oral contraceptives, steroids, antibiotics, and alcohol intake.15 Besides medications and alcohol, bacterial and viral infections such as HIV, tuberculosis, and Rickettsia have also been associated with the development of PH.15 More uncommon associations include malignancy such as HCC, cystic fibrosis, celiac disease, intestinal telangiectasia, portal vein aneurysms, Hodgkin's lymphoma, X-linked myotubular myopathy, hereditary haemorrhagic telangiectasia, and idiopathic thrombocytopaenic purpura.15272829 Despite the multiple reported associations, no reported association / causative factors have been found in up to 50% of cases.
Two histologic patterns of PH have been described in prior literature: the “parenchymal” type, consisting of irregular blood-filled cystic spaces without endothelial lining or fibrosis; and the “phlebectatic” type, comprising regular spherical spaces lined by endothelial cells or with a fibrous wall.20 It has been suggested that these two distinct morphologic appearances represent different parts of the same disease spectrum. The rupture of sinusoidal walls in the “parenchymal” type constitutes the early phase and the “phlebectatic” type is regarded as the end phase of PH.21 The histologic appearance of the lesions in this patient were largely compatible with the “parenchymal”-type lesions of PH. However, a rather unusual finding in this case was the presence of a dense meshwork of reticulin fibers within the blood-filled cavities, instead of the expected loss of reticulin fiber network due to breakdown of the sinusoidal walls. It might be postulated that this could represent an intermediate phase of PH, whereby the haemorrhagic lesions were undergoing resorption, although one would expect a peripheral granulation tissue response or evidence of an organizing thrombus if that were the case. Neither of these were present. Other histologic differential diagnoses considered were bacillary angiomatosis, haemorrhagic necrosis in a neoplastic hepatocellular lesion, a vasoproliferative neoplasm or other causes of sinusoidal dilatation and injury. They were excluded with the aid of special stains and immunohistochemistry.
The patient remains well today, having recovered fully from surgery. The management of her remaining disease remains difficult, and the risk and benefits of expectant management versus aggressive treatment must be weighed. Given her disease was not associated with any known causative factors and that she has already undergone a major resection, we chose to manage her left sided disease with regular imaging. This decision was made in conjunction with the patient. This case demonstrates that PH can masquerade as HCC and can be associated with a raised AFP. Clinical suspicion should be high in a patient, who presents with multiple lesions on imaging, with variable enhancement patterns across lesions. A detailed history of risk factors for PH should be sought.
Figures and Tables
Fig. 1
Computed tomography showing arterial phase with an arterial enhancing focus in segment 6 (A) and portal venous phase with hypodense lesion in the segment 6/7.
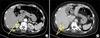
Fig. 2
Magnetic resonance imaging showing T2-weighted coronal section with multiple hyperintense lesions (A) and T2-weighted transverse cuts with multiple hyperintense lesions (B) and (C) in the right lobe.

Fig. 4
Microphotographs showing (A) blood-filled cavity in the hepatic parenchyma with no discernible endothelial lining or surrounding fibrosis and mild sinusoidal dilation at the periphery (Hematoxylin and Eosin stain, ×100) and (B) dense reticulin meshwork within the area of hemorrhage (reticulin stain, ×100).

Table 1
Laboratory results

AST, aspartate aminotransferase; ALT, alanine aminotransaminase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; HAV, hepatitis A virus; HBsAg, hepatitis B surface antigen; anti-HBc, antibody to hepatitis B core antigen; HCV, hepatitis C virus; PT, prothrombin time; INR, international normalization ration; aPTT, activated partial thromboplastin time; ANA, anti-nuclear antibody; AFP, alpha-fetoprotein
References
1. Okada S, Sato T, Okusaka T, Ishii H, Ikeda M, Nakasuka H, et al. Past exposure to hepatitis B virus as a risk factor for hepatocellular carcinoma in patients with chronic liver disease. Br J Cancer. 1998; 77:2028–2031.
2. Coppola N, Onorato L, Sagnelli C, Sagnelli E, Angelillo IF. Association between anti-HBc positivity and hepatocellular carcinoma in HBsAg-negative subjects with chronic liver disease: A meta-analysis. Medicine (Baltimore). 2016; 95:e4311.
3. Coppola N, Onorato L, Iodice V, Starace M, Minichini C, Farella N, et al. Occult HBV infection in HCC and cirrhotic tissue of HBsAg-negative patients: a virological and clinical study. Oncotarget. 2016; 7:62706–62714.
4. Huang CY, Wang ZW. Peliosis hepatis mimicking hepatic malignancy: a case report. J Dig Dis. 2013; 14:272–275.
5. Battal B, Akgun V, Sari S. Peliosis hepatis: one pathology, a thousand faces, and a clinical and radiological diagnostic challenge. J Dig Dis. 2014; 15:281–282.
6. Grønlykke L, Tarp B, Dutoit SH, Wilkens R. Peliosis hepatis: a complicating finding in a case of biliary colic. BMJ Case Rep. 2013; 2013:bcr2013200539.
7. Ferrozzi F, Tognini G, Zuccoli G, Cademartiri F, Pavone P. Peliosis hepatis with pseudotumoral and hemorrhagic evolution: CT and MR findings. Abdom Imaging. 2001; 26:197–199.
8. Iannaccone R, Federle MP, Brancatelli G, Matsui O, Fishman EK, Narra VR, et al. Peliosis hepatis: spectrum of imaging findings. AJR Am J Roentgenol. 2006; 187:W43–W52.
9. Leslie DF, Johnson CD, MacCarty RL, Ward EM, Ilstrup DM, Harmsen WS. Single-pass CT of hepatic tumors: value of globular enhancement in distinguishing hemangiomas from hypervascular metastases. AJR Am J Roentgenol. 1995; 165:1403–1406.
10. Kim EA, Yoon KH, Jeon SJ, Cai QY, Lee YW, Yoon SE, et al. Peliosis hepatis with hemorrhagic necrosis and rupture: a case report with emphasis on the multi-detector CT findings. Korean J Radiol. 2007; 8:64–69.
11. Gouya H, Vignaux O, Legmann P, de Pigneux G, Bonnin A. Peliosis hepatis: triphasic helical CT and dynamic MRI findings. Abdom Imaging. 2001; 26:507–509.
12. Torabi M, Hosseinzadeh K, Federle MP. CT of nonneoplastic hepatic vascular and perfusion disorders. Radiographics. 2008; 28:1967–1982.
13. Xiong WJ, Hu LJ, Jian YC, He Y, Zhou W, Guo XL, et al. Focal peliosis hepatis in a colon cancer patient resembling metastatic liver tumor. World J Gastroenterol. 2012; 18:5999–6002.
14. Alessandrino F, Felisaz PF, La Fianza A. Peliosis hepatis associated with hereditary haemorrhagic telangiectasia. Gastroenterol Rep (Oxf). 2013; 1:203–206.
15. Crocetti D, Palmieri A, Pedullà G, Pasta V, D'Orazi V, Grazi GL. Peliosis hepatis: Personal experience and literature review. World J Gastroenterol. 2015; 21:13188–13194.
16. Steinke K, Terraciano L, Wiesner W. Unusual cross-sectional imaging findings in hepatic peliosis. Eur Radiol. 2003; 13:1916–1919.
17. Kim SH, Lee JM, Kim WH, Han JK, Lee JY, Choi BI. Focal peliosis hepatis as a mimicker of hepatic tumors: radiological-pathological correlation. J Comput Assist Tomogr. 2007; 31:79–85.
18. Tsokos M, Erbersdobler A. Pathology of peliosis. Forensic Sci Int. 2005; 149:25–33.
19. Hayward SR, Lucas CE, Ledgerwood AM. Recurrent spontaneous intrahepatic hemorrhage from peliosis hepatis. Arch Surg. 1991; 126:782–783.
20. Yanoff M, Rawson AJ. Peliosis hepatis. An anatomic study with demonstration of two varieties. Arch Pathol. 1964; 77:159–165.
21. Zak FG. Peliosis hepatis. Am J Pathol. 1950; 26:1–15. incl 2 pl.
22. Khadilkar UN, Prabhu S, Sharma D. Peliosis hepatis presenting as hemoperitoneum. Indian J Med Sci. 2008; 62:236–237.
23. Choi SK, Jin JS, Cho SG, Choi SJ, Kim CS, Choe YM, et al. Spontaneous liver rupture in a patient with peliosis hepatis: a case report. World J Gastroenterol. 2009; 15:5493–5497.
24. Sommacale D, Palladino E, Tamby EL, Diebold MD, Kianmanesh AR. Spontaneous hepatic rupture in a patient with peliosis hepatis: A report of one case. Int J Surg Case Rep. 2013; 4:508–510.
25. Berzigotti A, Magalotti D, Zappoli P, Rossi C, Callea F, Zoli M. Peliosis hepatis as an early histological finding in idiopathic portal hypertension: A case report. World J Gastroenterol. 2006; 12:3612–3615.
26. Zafrani ES, Cazier A, Baudelot AM, Feldmann G. Ultrastructural lesions of the liver in human peliosis. A report of 12 cases. Am J Pathol. 1984; 114:349–359.
27. Mungan Z, Pinarbasi B, Bakir B, Gulluoglu M, Baran B, Akyuz F, et al. Congenital portal vein aneurysm associated with peliosis hepatis and intestinal lymphangiectasia. Gastroenterol Res Pract. 2009; 2009:479264.
28. Kleger A, Bommer M, Kunze M, Klaus J, Leithaeuser F, Wegener M, et al. First reported case of disease: peliosis hepatis as cardinal symptom of Hodgkin's lymphoma. Oncologist. 2009; 14:1088–1094.
29. Motoki T, Fukuda M, Nakano T, Matsukage S, Fukui A, Akiyoshi S, et al. Fatal hepatic hemorrhage by peliosis hepatis in X-linked myotubular myopathy: a case report. Neuromuscul Disord. 2013; 23:917–921.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download