Abstract
Backgrounds/Aims
Surgical resection, such as pancreaticoduodenectomy (PD), is used for treatment of benign periampullary tumors, but high morbidity and mortality resulting from PD can be a huddle. The aim of this study is to suggest a safe and less invasive procedure for treatment of benign periampullary tumors.
Methods
From January 2001 to September 2016, 31 patients with ampulla of Vater (AOV) tumors were reviewed retrospectively. Patients who were confirmed with malignancy through biopsy were excluded, except for one patient with malignancy and multiple underlying diseases. To investigate the safety and availability of transduodenal ampullectomy (TDA), TDA and endoscopic papillectomy (EP) were compared.
Tumors originating from the ampulla of Vater (AOV) may be either benign neoplasms or malignant. Benign neoplasms are rare, representing less than 10% of periampullary neoplasms.1 The most common benign lesions are adenomas.23 Ampullary adenomas may possibly progress to carcinoma.4
There are three ways to remove periampullary adenomas: pancreaticoduodenectomy (PD), transduodenal ampullectomy (TDA), or endoscopic papillectomy (EP). Indications for surgical resection are an adenoma greater than 2 cm in diameter, evidence of lymph node involvement, and evidence of ingrowth of adenoma into the bile or pancreatic duct.5 However, high morbidity and mortality due to aggressive resection remain an issue. Before PD was introduced by Whipple in 1899, Halstead first introduced TDA. However, due to high recurrent rate in AOV cancers, interest in TDA has retreated without much response.67 Consequently, a less invasive procedure, EP, first described in the late 1980s,89 has been developed due to high morbidity and mortality associated with surgical resection. Only recently have EP and TDA become common procedures for treatment of benign ampullary tumors.
Since there are no clear guidelines for treatment of periampullary tumors,10 the purpose of this study was to suggest a safe and minimally invasive method to radically remove AOV tumors by comparing TDA with EP.
From January 2001 to September 2016, 31 patients with AOV tumors were reviewed retrospectively. Patients confirmed with malignancy through biopsy were excluded from this study. Patients underwent a preoperative lab test, esophagogastroduodenoscopy (EGD) exam, preoperative biopsy, computed tomography (CT), endoscopic ultrasonography (EUS), and magnetic resonance imaging (MRI). Endoscopic retrograde cholangiopancreatography (ERCP) was conducted to confirm ductal involvement before conducting EP. Since there are no clear guidelines for treatment of ampullary tumors, EP was conducted for benign ampullary tumors measuring less than 1 cm in diameter, and TDA was conducted for tumors greater than 1 cm in diameter. Pancreatic and biliary stents were inserted into patients that underwent EP to prevent postoperative complications such as pancreatitis and stenosis. Lab tests and chest pain film radiography were routinely conducted to check for complications after EP. Five days after EP, EGD was conducted to remove the stent and to investigate possible ulcerative lesions or bleeding sites, often appearing during the early recovery period after EP.1112
For patients unable to undergo EP, TDA was conducted as initial treatment. TDA was conducted under two conditions: tumor size greater than 1 cm in diameter; and preoperative biopsy confirming tumor as benign with no evidence of lymph node involvement through image studies. Intraoperative frozen sections for pancreatic duct, bile duct, and tumor margins were routinely submitted to pathology. An upper midline or extended right subcostal incision was used. When colonic hepatic flexure was mobilized, the Kocher maneuver was conducted to expose the posterior aspect of the second portion of the duodenum. A 5–6 cm longitudinal duodenotomy was conducted along the anterior wall where mass was palpated. After identifying the common bile duct (CBD) and pancreatic duct, full-thickness excision of the entire grossly visible tumor was conducted until the pancreas head tissue was exposed. To prevent stricture in the pancreatic duct, a plastic tube measuring approximately 2–3 cm in length was inserted, and tagging sutures were applied with 5-0 absorbable polydioxanone (PDS). Both ducts were reinforced with interrupted suture technique on the wall of the duodenum using 5-0 PDS. To avoid narrowing of the lumen, the duodenum was closed in a transverse direction. A closed suction drain was inserted routinely in a standard manner. Fascial and skin closures were conducted in usual fashion.
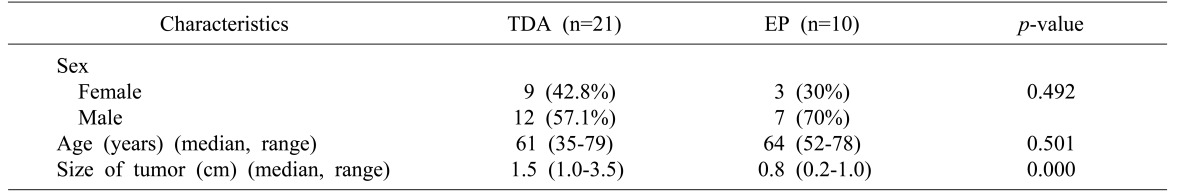
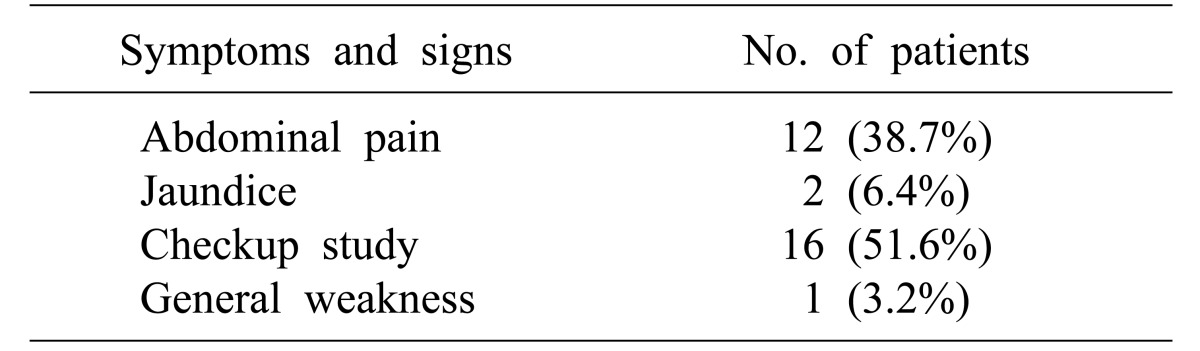
Preoperative symptoms and patient characteristics, including size of the tumor, postoperative outcomes, ERCP biopsy results, frozen biopsy results during the operation, and final pathological findings, were compiled.
Statistical analysis was conducted using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). The significance of differences between groups in estimated blood loss, mean hospital admission time, and follow-up characteristics was assessed by Student's t-test. The total number of complications, transfusions, and recurrence rates was assessed by chi-squired test. A p-value<0.05 was an indication of statistical significance.
This study is based on a retrospective review of 31 patients that underwent TDA or EP for ampullary tumors. Of these patients, 16 were diagnosed during routine health checkup without symptoms, except for one patient presented with recurrence. Twelve patients reported abdominal pain, two had jaundice, and one had general weakness (Table 1). Twenty-one TDAs and 10 EPs were conducted. There was no significant difference in the total number of complications between the TDA group and EP group (p=0.145). Resection margins were negative in both groups. There was no recurrence in patients with TDA, while one patient experienced recurrence after EP. Four patients that underwent TDA have malignancy even though intraoperative analysis of frozen sections suggested tumors were benign. There were no cases of converted operation from TDA to PD. In the TDA group, nine patients were female and 12 were male, the median patient age was 61 years (range: 35–79), and the median tumor size was 1.5 cm (range: 1.0–3.5). In the EP group, three patients were female and seven were male, the median patient age was 64.4 years (range: 52–78), and the median tumor size was 0.8 cm (range: 0.2–1.0) (Table 2).
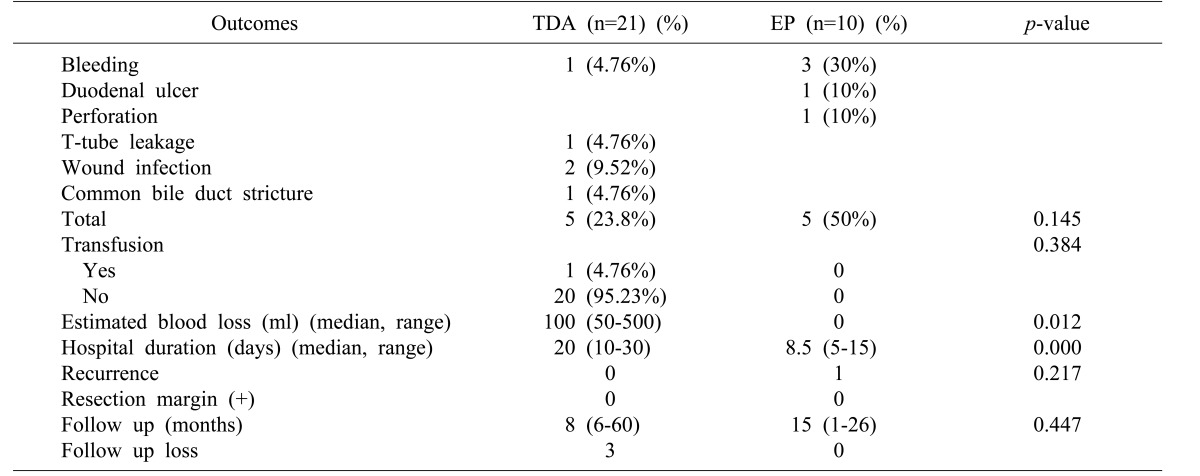
There was no significant difference in the total number of complications between the TDA group and EP group (p=0.145). Five cases of complications were observed in the TDA group: one patient that had a transfusion of five packs of red blood cells due to postoperative bleeding, one case of T-tube leakage, two cases of wound infection, and one case of common bile duct stricture. Three cases of bleeding, one case of duodenal ulceration, and one case of duodenal perforation were observed in the EP group. Median estimated blood loss was 100 ml (range: 50–500) in the TDA group. Duration of hospital stay was longer in the TDA group compared with the EP group (20 vs. 8.5 days, respectively). Resection margins were negative in both groups. One patient experienced recurrence after EP, although the procedure had been conducted in a different institute several years before admission to the present hospital (Table 3).
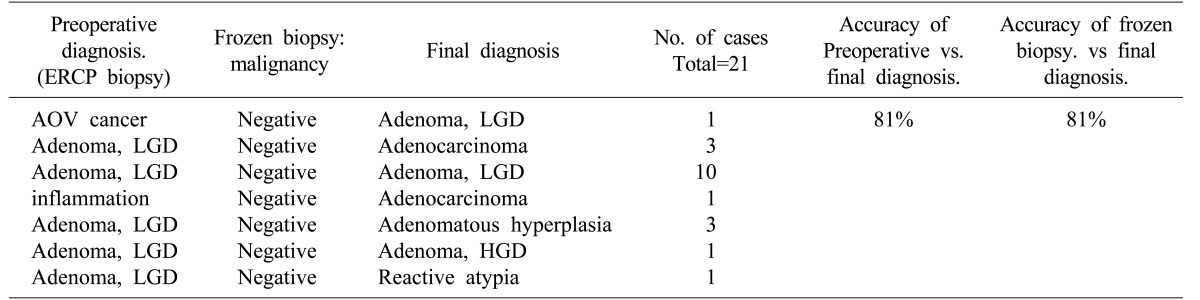
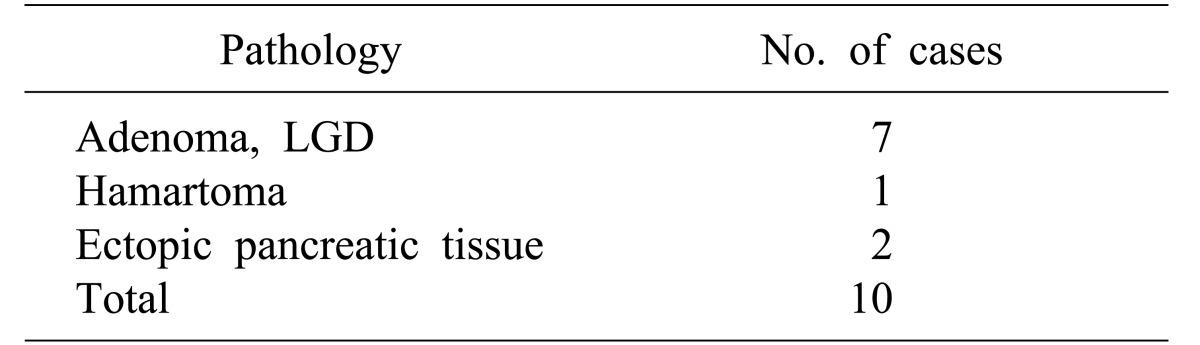
There were seven cases of low-grade adenoma, one case of hamartoma, and two cases of ectopic pancreatic tissue in the EP group (Table 4). In the TDA group, 11 cases of low-grade adenoma, five cases of adenocarcinoma, three cases of adenomatous hyperplasia, one case of high-grade adenoma, and one case of reactive atypia were verified by permanent pathology. One patient diagnosed with adenocarcinoma prior to TDA was previously mentioned above. Accuracy of preoperative biopsy versus final pathological assessment was 81%, and that of intraoperative frozen section versus final pathological results was also 81% (Table 5).
Development of surveillance studies and accurate methods for detection of malignancy has yielded innovative and less invasive techniques. For reasons mentioned above, clinicians have often conducted TDA and EP in recent years even though there are no definite criteria indicating patients should have either procedure performed.1013 For patients lacking an indication for EP, TDA may be an alternative method for removing ampullary tumors.
EP is a less invasive technique, with low morbidity and mortality.4 Mean length of stay (LOS) in the hospital for EP is comparatively short, a benefit for the patient. Despite advantages, frequent endoscopic checkups are required after EP, and false negative results resulting from inadequate margins may be a drawback.141516 Procedural outcomes may vary depending on the type of institution at which the operation is conducted and if surgeons involved have previously conducted the procedure. Presence of obstacles and inadequate excision of tissue may occur due to limited visual space. The AOV must be resected in full layers with negative margins to prevent remnant tumors.1718 EP should be considered in patients with smaller lesions that do not contain carcinoma and patients that are poor surgical candidates. Tumor size may be an additional issue to consider if choosing the optimal method for a patient. A previous study revealed that adenomas with high-grade dysplasia (HGD) or cancer were 1.8 and 2.0 cm on average, respectively, whereas those with low-grade dysplasia were 1.3 cm on average.19 One of our cases had duodenal perforation after EP, that could have developed into peritonitis. This indicates that a simple procedure may become critical; therefore, close monitoring is recommended in all cases.
TDA may be an alternative treatment to aggressive surgeries such as PD. Although there are currently no clear guidelines for election of TDA, it may be considered under following conditions: adenoma greater than 2 cm in diameter; no evidence of lymph node involvement; and no evidence of ingrowth of adenoma in the bile or pancreatic duct. According to the International Classification of Disease for Oncology (ICD-O), defined by the World Health Organization (WHO), adenoma with HGD may be considered as carcinoma in situ (Tis). In a Tis neoplasm, the lamina propria is intact with no lymph node metastasis.2021 This forms the basis of the possibility of using TDA for ampullary Tis neoplasms. However, there is no significant data regarding patient outcomes from these surgeries.
Converting from TDA to PD is possible with intraoperative frozen biopsy. It may prevent unnecessary invasive surgery for benign tumors in the AOV. TDA allows complete resection compared to EP of the AOV.1722 There was one case in which malignancy was suspected on preoperative analysis, but the tumor was benign in final pathological analysis. Conversely, if preoperational diagnosis is not accurate, there is a risk of misdiagnosis of benign tumors, and of missed cancer. Therefore, an accurate diagnosis with intraoperative lymph node biopsy is critical. In this study, four patients initially diagnosed with adenoma have adenocarcinoma in final pathological analysis; however, no evidence of recurrence was revealed during mean patient follow-up of 24 months. Another patient diagnosed with adenocarcinoma via preoperational biopsy underwent TDA due to the presence of other underlying diseases. Although it was not necessary to conduct in concert with TDA, palliative chemotherapy was conducted, and there was no recurrence in 60 months.
PD is the definitive approach for radical resection of ampullary tumors. It lowers risk of recurrence and the need for post-procedural surveillance endoscopy. Recurrence rates for ampullary adenomas after PD are nearly zero. However, high morbidity and mortality rates may be an obstacle with such an aggressive surgical resection.
There are several limitations to this study. 1) The study was conducted by two separate departments. Since no consensus has been reached regarding the definition of a 1.0 cm benign tumor, the Internal Medicine Department conducted EP, and the Department of Surgery conducted TDA. 2) Three cases from the TDA group were lost during the follow-up period, and the median follow-up duration was less than 24 months; 8 months (range: 6–60) in the TDA group and 15 months (range: 1–26) in the EP group. 3) Sample sizes in both groups were too limited to be analyzed statistically. Further evaluation with a lengthier follow-up period is required. 4) An evidence-based study with a larger sample size is required to conclude that TDA is safe and recommended in early-stage cancers of the AOV. 5) There may be an information bias or a selection bias. To investigate safety of TDA in treatment of Tis, similar sample sizes in TDA and PD groups should be compared.
EP prevents unnecessary abdominal exploration. However, patients that cannot undergo EP must face the risk of aggressive surgical resection. This study suggests that TDA is as safe as EP for benign periampullary tumors. TDA may be considered especially for patients requiring precise resection or for patients with remnant or recurrent tumors after EP.
References
1. Branum GD, Pappas TN, Meyers WC. The management of tumors of the ampulla of Vater by local resection. Ann Surg. 1996; 224:621–627. PMID: 8916877.

2. Treitschke F, Beger HG. Local resection of benign periampullary tumors. Ann Oncol. 1999; 10(Suppl 4):212–214. PMID: 10436825.

3. Allgaier HP, Schwacha H, Kleinschmidt M, Thimme R, Schöffel U, Blum HE. Ampullary hamartoma: A rare cause of biliary obstruction. Digestion. 1999; 60:497–500. PMID: 10473976.

4. Stolte M, Pscherer C. Adenoma-carcinoma sequence in the papilla of Vater. Scand J Gastroenterol. 1996; 31:376–382. PMID: 8726307.

5. de Castro SM, van Heek NT, Kuhlmann KF, Busch OR, Offerhaus GJ, van Gulik TM, et al. Surgical management of neoplasms of the ampulla of Vater: local resection or pancreatoduodenectomy and prognostic factors for survival. Surgery. 2004; 136:994–1002. PMID: 15523392.

6. Halsted WS. Contributions to the surgery of the bile passages, especially of the common bile duct. Boston Med Surg J. 1899; 141:645–654.
7. Whipple AO, Parsons WB, Mullins CR. Treatment of carcinoma of the ampulla of vater. Ann Surg. 1935; 102:763–779. PMID: 17856666.
8. Shemesh E, Nass S, Czerniak A. Endoscopic sphincterotomy and endoscopic fulguration in the management of adenoma of the papilla of Vater. Surg Gynecol Obstet. 1989; 169:445–448. PMID: 2683151.
9. Lambert R, Ponchon T, Chavaillon A, Berger F. Laser treatment of tumors of the papilla of Vater. Endoscopy. 1988; 20(Suppl 1):227–231. PMID: 3168951.

10. Standards of Practice Committee. Adler DG, Qureshi W, Davila R, Gan SI, Lichtenstein D, et al. The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2006; 64:849–854. PMID: 17140885.

11. Binmoeller KF, Boaventura S, Ramsperger K, Soehendra N. Endoscopic snare excision of benign adenomas of the papilla of Vater. Gastrointest Endosc. 1993; 39:127–131. PMID: 8495831.

12. Desilets DJ, Dy RM, Ku PM, Hanson BL, Elton E, Mattia A, et al. Endoscopic management of tumors of the major duodenal papilla: Refined techniques to improve outcome and avoid complications. Gastrointest Endosc. 2001; 54:202–208. PMID: 11474391.

13. Winter JM, Cameron JL, Olino K, Herman JM, de Jong MC, Hruban RH, et al. Clinicopathologic analysis of ampullary neoplasms in 450 patients: implications for surgical strategy and long-term prognosis. J Gastrointest Surg. 2010; 14:379–387. PMID: 19911239.

14. Lee SY, Jang KT, Lee KT, Lee JK, Choi SH, Heo JS, et al. Can endoscopic resection be applied for early stage ampulla of Vater cancer? Gastrointest Endosc. 2006; 63:783–788. PMID: 16650538.

15. Seewald S, Omar S, Soehendra N. Endoscopic resection of tumors of the ampulla of Vater: how far up and how deep down can we go? Gastrointest Endosc. 2006; 63:789–791. PMID: 16650539.

16. Han J, Kim MH. Endoscopic papillectomy for adenomas of the major duodenal papilla (with video). Gastrointest Endosc. 2006; 63:292–301. PMID: 16427938.

17. Bellizzi AM, Kahaleh M, Stelow EB. The assessment of specimens procured by endoscopic ampullectomy. Am J Clin Pathol. 2009; 132:506–513. PMID: 19762527.

18. Chijiiwa K, Yamashita H, Kuroki S. Wide ampullectomy for patients with villous adenoma of duodenal papilla and follow-up results of pancreaticobiliary tract. Int Surg. 1994; 79:178–182. PMID: 7928158.
19. Hornick JR, Johnston FM, Simon PO, Younkin M, Chamberlin M, Mitchem JB, et al. A single-institution review of 157 patients presenting with benign and malignant tumors of the ampulla of Vater: management and outcomes. Surgery. 2011; 150:169–176. PMID: 21801957.

20. Gassler N, Knüchel R. Tumors of Vater's ampulla. Pathologe. 2012; 33:17–23. PMID: 22293786.
21. Sharma P, Montgomery E. Gastrointestinal dysplasia. Pathology. 2013; 45:273–285. PMID: 23442738.

22. Grobmyer SR, Stasik CN, Draganov P, Hemming AW, Dixon LR, Vogel SB, et al. Contemporary results with ampullectomy for 29 "benign" neoplasms of the ampulla. J Am Coll Surg. 2008; 206:466–471. PMID: 18308217.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download