INTRODUCTION
Gallstone disease poses significant health problems. In Asia, westernization of the diet and socioeconomic status have changed the patterns of gallstone disease.
1 Cholecystectomy is the treatment of choice for symptomatic gallstones. After the adoption of noninvasive laparoscopic cholecystectomy, the number of cholecystectomies has increased worldwide. Stomach cancer is the fifth most common cancer in the world—and the highest incidences have been in East Asia, China, Eastern Europe, Latin America, and the Caribbean.
2
In healthy individuals, 75–80% of hepatic bile enters the gallbladder and only 20–25% of bile directly enters into the duodenum. In cases of chronic calculous cholecystitis, 30–40% of hepatic bile enters the gallbladder and 60–70% of hepatic bile passes into the duodenum (due to the decreased rate of water absorption in the gallbladder wall). Following cholecystectomy, 100% of the hepatic bile enters the duodenum (
Fig. 1).
3
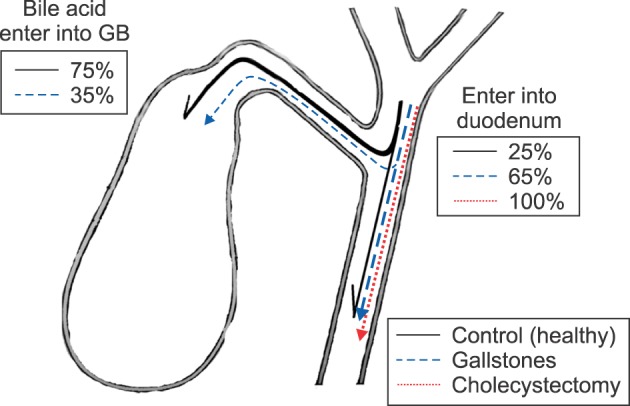 | Fig. 1Passage of hepatic bile acid into the gallbladder and the duodenum. GB, gallbladder.
|
During the process of enterohepatic circulation, anaerobic bacteria promote 7α-dehydroxylation of hydrophilic primary bile acids (cholic acid and chenodeoxycholic acid), converting them into hydrophobic secondary bile acids (deoxycholic acid [DCA] and lithocholic acid).
Depending on the concentration, hydrophobic secondary bile acids can cause cholestasis and necrosis or promote apoptosis and DNA damage. Furthermore, DCA is not only mutagenic but carcinogenic. Taken together, the evidence suggests that the human gallbladder fulfills a protective function. The gallbladder decreases the formation of secondary hydrophobic hepatotoxic bile acids by accumulating primary bile acids thereby, protecting the liver and the mucosa of the stomach.
4
Based on the biological mechanism described above, we hypothesized that gallstones, cholecystectomy would be associated with an increased risk of stomach cancers, and evaluated the association of gallstones, cholecystectomy with stomach cancer in a hospital-based case-control study.
Go to :

MATERIALS AND METHODS
Study sample
A total of 99 patients newly diagnosed with stomach cancers were enrolled in this study. Between April 1994 and December 2015, we identified 5,566 patients whose records contained a cholecystectomy code (International Classification of Disease, Ninth Revision). The gallstones group consisted of 6,891 patients who had a diagnosis of gallstones and were not cholecystectomized.
To reduce the effect of selection bias, we excluded stomach cancers, which appeared during the first year of follow-up either cholecystectomy or after a diagnosis of non-surgically-treated gallstones. This was done for the detection of stomach cancer cases identified only because of gallstones and cholecystectomy.
Control patients who voluntarily visited the Dong-A University Hospital Health Care System, for a health check-up between June 2007 and December 2015 were enrolled. As a part of these health care programs, patients were routinely screened with ultrasonography. Of the 37,236 initially enrolled patients, 2,525 foreign patients or gallstone patients were excluded. Therefore, 34,711 control patients were included in this analysis. We retrospectively reviewed the patient's medical records.
Immunohistochemical studies
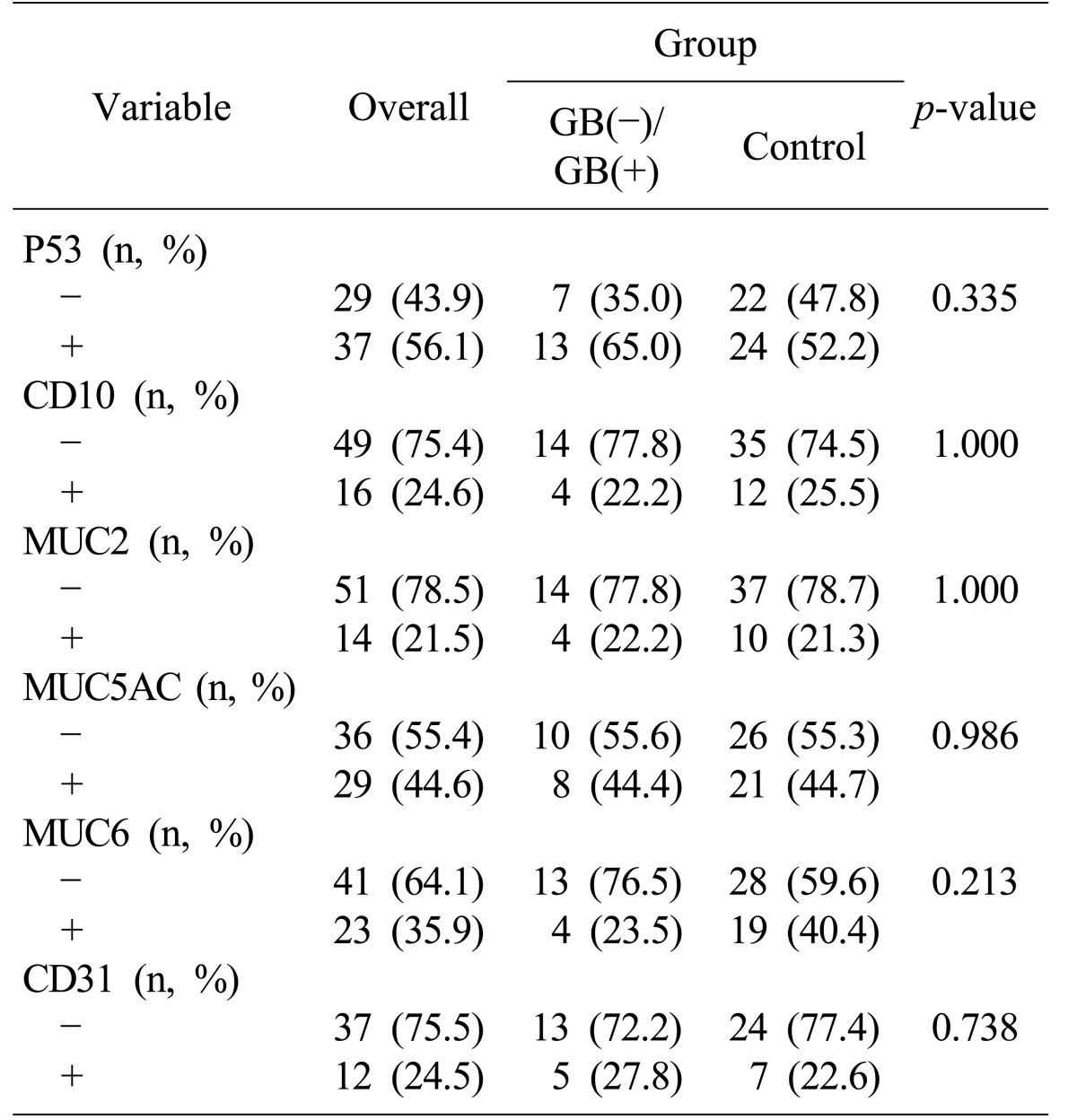
Immunohistochemical chemistry was used as diagnostic aids, prognostic or predictive indicators, or histogenetic probes. The p53 protein was detected for genomic stability. CD10 and MUC2 mucin were used as biomarkers for the identification of intestinal metaplasia. MUC5AC and MUC6 mucins were used as biomarkers for the identification of gastric goblet epithelium. CD31 was used as a biomarker for the identification of endothelial cells.
Endoscopic finding
We determined the level of each stomach cancer (cardia or non-cardia), along with the location of each (posterior, greater curvature, anterior, lesser curvature). Usually, due to the flow and gravity of bile juice, duodenogasrtic-bile-reflux-induced stomach cancers were located in the posterior, greater curvature of the stomach with greater frequency than in the anterior, and lesser curvature of the stomach.
Statistical analysis
Variables were summarized by frequency and percentage for the categorical data. A mean±standard test or Fisher's exact test was used for categorical data. An independent t-test was used for numeric data. Overall survival (OS) was estimated using Kaplan-Meier curves.
Survival curves were compared between groups using the log-rank test. Multivariate analyses, using Cox regression, were performed in order to identify prognostic factors, which are independently related to overall survival.
All statistical analyses were carried out using SPSS 22.0 (IBM Corp., Armonk, NY) and MedCalc 11.6.0 (MedCalc, Mariakerke, Belgium) statistical software. P values of less than 0.05 were considered to be statistically significant. By its nature, this study was explorative. Therefore, no adjustment for multiple testing was applied.
Go to :

RESULTS
The demographic characteristics of stomach cancers are shown in
Table 1.
Table 1
Baseline characteristics of the stomach cancer patients
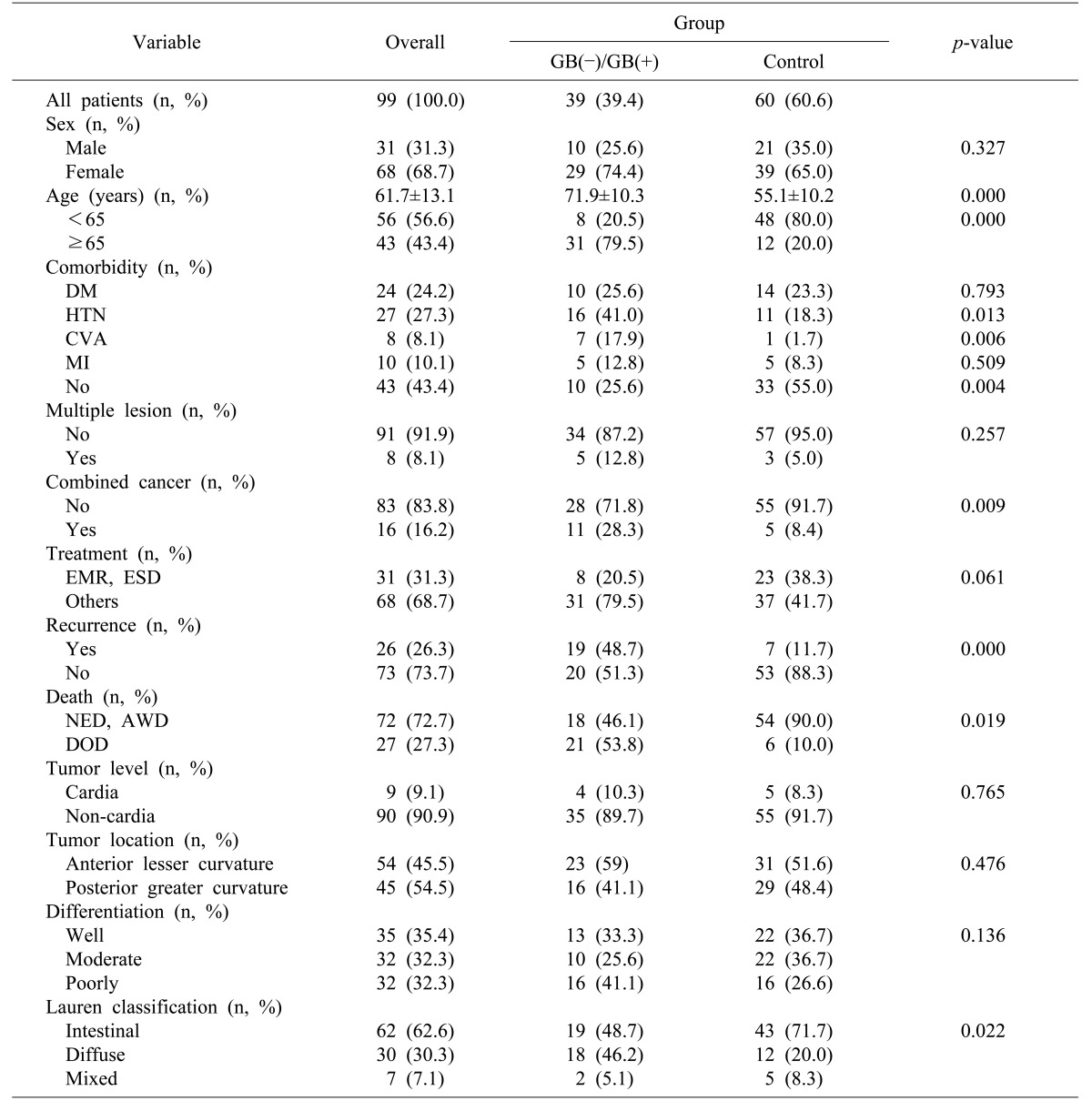

The gender distribution was 31.1% male and 68.7% female. The mean age was 61.7±13.1 years. The patients in both the gallstone group and cholecystectomy group were older than those in the control group (
p=0.000). The median follow-up time was 53 months. The overall incidence rate of stomach cancer was higher in the gallstone and cholecystectomy groups than in the control group (
p=0.003) (
Fig. 2).
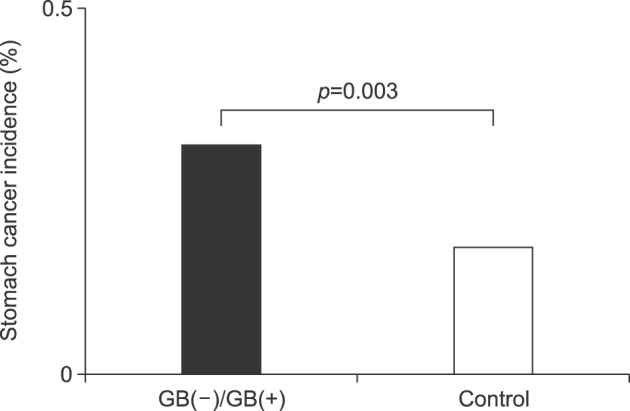 | Fig. 2Comparison of stomach cancer incidence between the groups. GB(−), cholecystectomy; GB(+), gallstones.
|
The median overall survival rate was shown to be worse in the gallstone and cholecystectomy group than in the control group (
p=0.000) (
Fig. 3).
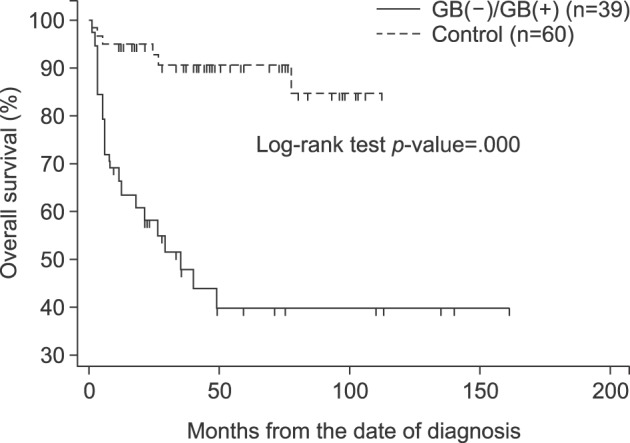 | Fig. 3Overall patient survival rate, according to the groups. GB(−), cholecystectomy; GB(+), gallstones.
|
The prevalence of comorbidities—especially hypertension and cerebrovascular comorbidities—was greater in the gallstone and cholecystectomy groups (p=0.004).
The incidence of different types of combined malignancies (4 colon cancers, 4 lung cancers, 3 thyroid cancers, 3 pancreatic cancers, and 2 hepatocellular carcinomas), was more frequent in the gallstone and cholecystectomy groups than in the control group (p=0.009).
The level of stomach cancer (cardia or non-cardia) (p=0.765) and its location (posterior, greater curvature versus anterior, lesser curvature) (p=0.476), showed insignificant statistical differences. The differentiation of stomach cancer showed an insignificant statistical difference between the groups; but in the Lauren classification, the intestinal type was more frequent in the control group (p=0.022).
Immunohistochemical studies P53, CD10, MUC 2, M5AC, MUC6, and CD 31 showed insignificant statistical differences between gallstone and cholecystectomy groups, and the control group (
Table 2).
Table 2
Immunohistochemical analysis of the stomach cancer patients

Treatment modalities for stomach cancer such as endoscopic mucosal resection and endoscopic submucosal dissection—along with other modalities—were compared; but no statistical differences were observed between the groups (p=0.061).
Hazard ratios (HR) and 95% confidence intervals (CI) were calculated for death, using a multivariate Cox regression model.
The HR for OS in the gallstone and cholecystectomy patients as compared with that in control patients is 6.656 (p=0.003: 95% CI, 1.94–22.80) Also, tumor (T)-stage was found to affect OS statistically (HR=9.85: 95% CI, 3.09–31.39: p=0.000).
Patients with stomach cancers in the posterior, greater curvature location of the stomach showed worse OS than did those with cancers in the anterior, lesser curvature location of the stomach (HR=0.30:
p=0.017: 95% CI, 0.11-0.80) (
Table 3).
Table 3
Overall survival in gallstones, after cholecystectomy group patients as compared with subjects in the control group
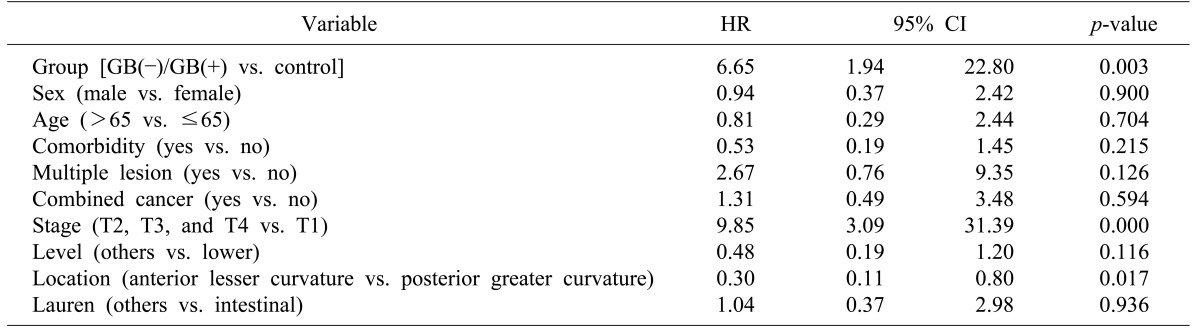

The gallstones and cholecystectomy group patients showed advanced stage cancer. Also, cancer in the posterior, greater curvature location of the stomach—which might be caused by duodenogastric bile reflux—results in a poor survival rate (p=0.017).
Go to :

DISCUSSION
The association of the gallstone and cholecystectomy group patients with stomach cancer suggests that exposure to bile may be an important carcinogenic mechanism. In the gallstone and cholecystectomy groups, the overall incidence of stomach cancer was higher in this study's series—a finding which was consistent with other results.
567 By contrast, other studies yielded data that conflicted with these results.
89
The burdensome challenge of the prevalence of gallstones in western countries (ranging from 5.9% to 21.9% in the general population) has increasingly become a problem. A genetic link to cholesterol (30% of the genetic component being accounted for by “thrifty” genes) has been postulated and the associations among diabetes, obesity, and gallstones most likely come about through the metabolic syndrome.
10
There has been a gradual increase in the incidence of gallstones in conjunction with the westernization of Korean lifestyle. As similar to that of other Asian countries, the prevalence of gallstones in the Korean population was 6.1%, and patients who underwent cholecystectomy had a 35% higher prevalence of non-alcoholic fatty liver disease.
111213 Chronic liver disease is a well-known risk factor for gallstones, diabetes mellitus, hepatitis B/C, and liver cirrhosis. Other risk factors for gallstones include certain drugs, total parenteral nutrition, fasting, and Crohn's disease.
High exposure to bile acids is prevalent among individuals who have a high dietary fat intake. High bile acid exposure increases the production of reactive oxygen species, reactive nitrogen species, increased DNA damage, increased mutation, and reduced apoptosis upon chronic exposure. It is very likely that the same risk factors for gallstones and cancer—such as obesity, diet, ethnicity, family history, and cigarette smoking—coexist in patients. Unless such confounders are adjusted for, it is difficult to conclude that the risk is purely an effect of cholecystectomy alone.
The proposed mechanism for increased risk of stomach cancer in gallstones, cholecystectomy group is through alteration of bile flow, increased exposure, alteration of bile salts, and subsequent alteration of metabolic hormone levels. Bile is not only stored in the gallbladder but is also concentrated, so that the biliary output to the gut is approximately halved in the presence of a functioning gallbladder. After cholecystectomy, a large volume of bile can overload the clearing capacity of the proximal duodenum and thereafter cause duodenogastric reflux. In cholecystectomy, all the bile secreted from the liver drains through the sphincter of Oddi into the duodenum, producing a continuous flow, and resulting in increased transcapillary bile flow and a CBD emptying rate. This increased and continuous bile flow can lead to a reflux of bile into the stomach and the esophagus.
Post-cholecystectomy syndrome is characterized by the persistence of symptoms or an occurrence of new symptoms following cholecystectomy. Sphincter of Oddi dysfunction is one of the most important symptoms following cholecystectomy and may be caused by injury of the direct neural pathway between the gallbladder and duodenum—as has been demonstrated in a Suncus murinus, whole-mount immunohistochemical study.
14
Some studies have suggested that the elimination of the gallbladder reservoir function following cholecystectomy, causes a change in the pattern of the bile flow into the duodenum, potentially increasing bile reflux into the stomach. One of the bile acids may be weak mutagen, whose cumulative effect could result in carcinogenesis after many years of exposure. Supporting evidence indicates that bile acids cause DNA damage and induce frequent apoptosis. After repeated apoptosis, the capability for the development of stomach cancer and esophageal adenocarcinoma and their actual documented incidences increase.
15161718 However, other studies have demonstrated that cholecystectomy does not result in either increased bile reflux into the stomach or increased gastroesophageal reflux.
19 The gallbladder regulates bile acid homeostasis, so a significant alteration in bile acid metabolite following cholecystectomy may alter glucose and lipid metabolism. Cholecystectomy increases enterohepatic circulation cycles and leads to greater enterohepatic circulation of estrogen, progesterone, and their active metabolites which cause inflammation, stimulate fat accumulation, lead to cholestasis, and induce breast cancer. Also, elevated circulating levels of cholecystokinin (CCK) have been identified and CCK has been shown to stimulate growth of human pancreatic cells.
202122 In a comprehensive review, no strong evidence was observed for association between cholecystectomy and subsequent development of cancers of the esophagus, pancreas, small bowel, and colon.
23
The function of P53 in response to DNA damage includes inducing cell cycle arrest, facilitating the DNA repair process, and promoting apoptosis. Thus, a reduction in P53 by DCA should increase genomic instability. DCA has also been shown to cause degradation of P53.
24 CD10 and MUC2 are useful for the identification of intestinal metaplasia. MUC5AC and MUC6 are useful for the identification of gastric goblet epithelium. CD 31 is useful for identification of the endothelial cell. In this study, P53, CD10, MUC 2, M5AC, MUC6, and CD 31, showed only insignificant statistical differences between the gallstone, cholecystectomy, and control groups.
The question of whether prophylactic cholecystectomy during gastrectomy for cancers is needed remains a controversial issue. The reason for prophylactic cholecystectomy is the incidence of gallstones after gastrectomy was considerably higher than was the prevalence of gallstones detected during the health examination in Korea (2.3–4.9%).
2526 The main pathophysiologic mechanism is considered to be an interruption of the anterior hepatic branch of the vagus nerve—a condition which leads to bile juice stagnation. So, is prophylactic cholecystectomy needed? On the contrary, after gastrectomy, most patients—at least 75%—will not have gallstone disease and may possibly experience a functional digestive problem as a result of unnecessary cholecystectomy having been performed.
27 Our results lend some support to the decision to avoid prophylactic cholecystectomy for the risk of stomach cancer, if indication for the surgery is uncertain.
This study provides more compelling results than do previous studies. Firstly, this study is a large-scale, hospital-based, case-control study. This study was based on a control group representative of the general population (considering the nature of the health screenings). Secondly, this study is not merely an epidemiological study, but a clinical study based on endoscopic findings, imaging results, immunohistochemical tests, and survival analyses for members of the Asian population. However, there are some limitations of this study. Firstly, the case-control design is more susceptible to recall and selection bias than is a cohort design. Secondly, retrospective studies such as this one generally tend to be lower in statistical quality than the findings of randomized controlled trials.
In conclusion, we suggest that there sould be an awareness of the possibility of an increased risk for developing stomach cancer in gallstones, cholecystectomy patients, which might be induced by duodenogastric bile reflux. Moreover, as the characteristics of the stomach cancers were only detected once they had already progressed to an advanced stage, the survival rate was worse than for those in the control group. Therefore, close follow-up strategies for the early detection of stomach cancer are recommended in caring for these patients. Further studies are needed regarding the linkage between gallstones, cholecystectomy, and stomach cancer.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download