Abstract
Backgrounds/Aims
Postoperative diaphragmatic hernia, following liver resection, is a rare complication.
Methods
Data of patients who underwent major hepatectomy for liver tumors, between 2011 and 2015 were retrospectively reviewed. The literature was searched for studies reporting the occurrence of diaphragmatic hernia following liver resection.
Results
Diaphragmatic hernia developed in 2.3% of patients (3/131) with a median delay of 14 months (4-31 months). One patient underwent emergency laparotomy for bowel obstruction and two patients underwent elective diaphragmatic hernia repair. At last follow-up, no recurrences were observed. Fourteen studies including 28 patients were identified in the literature search (donor hepatectomy, n=11: hepatectomy for liver tumors, n=17). Diaphragmatic hernia was repaired emergently in 42.9% of cases and digestive resection was necessary in 28.5% of the cases. One patient died 3 months after hepatectomy, secondary to sepsis, from a segment of small bowel that perforated into the diaphragmatic hernia.
Right-sided major liver resection (LR) is considered a technically complex liver resection.12 The right liver mobilization from the diaphragm may be cumbersome particularly, for tumors with invasion or severe adhesion of the diaphragm. This may lead to en-bloc resection of the diaphragm or diaphragm opening, when technical difficulty is encountered during right liver mobilization.
Diaphragmatic hernia (DH) is a rare complication following LR. After right-sided major LR, the large surface area of the right diaphragm previously covered by the right liver allows the right colon and small bowel to migrate into the infra-diaphragmatic fossa. Traumatic diaphragmatic defect and diaphragm resection have been described as risk factors for DH.34 DH are classified as congenital and acquired. Congenital DH results from abnormal development of the diaphragm during embryogenesis and is symptomatic, during the first day of life.5 Acquired DH is often traumatic in origin (blunt or penetrating injury), with an incidence ranging from 2.6% to 3.7%.3 DH has been rarely reported after LR. The literature review revealed 18 cases and 10 cases of DH following LR, for liver tumors and in the setting of living liver donor, respectively.46789101112131415161718
The aim of this study was to report our experience, to review the literature, and to explore the underlying potential mechanisms of DH in the setting of LR for tumors.
From January 2011 to February 2015, 131 elective major right-sided LRs were performed for benign and malignant tumors at Henri Mondor Hospital (Créteil, France). Of the cases identified from a maintained database, 3 (2.3%) patients developed postoperative DH, following LR. We reviewed the patient demographics, operative details, circumstances, and delay of DH.
Hepatic resections were performed using an open or laparoscopic approach in 117 and 14 cases, respectively.1920 A conventional approach or an anterior approach was used, according to the localization of the tumor and the preference of the surgeons. The conventional approach consists of performing full mobilization of the right liver before parenchyma transection. The anterior approach consists of performing liver parenchyma transection before the full mobilization of the right liver.21 Mobilization of the right liver includes a sectioning of the right triangular ligament and right diaphragmatic attachments to the right liver. This step was performed using either a monopolar cautery or scissors. Hemostasis of the right diaphragm was done using the bipolar sealer, bipolar coagulation, or monopolar cautery. Hemostasis and biliostasis were achieved by bipolar coagulation, a bipolar irrigated sealer (Aquamantys®) since 2013, sutures and metallic clips. At the end of transection plane, an observation of 20 minutes was performed to be sure that hemostasis was achieved. At least one abdominal drain was placed close to the transected surface in all patients.
After discharge, all patients were followed with a classic screening protocol including a thoracic and abdominal computed tomography, routine biological tests, and tumor markers every 3 months during the first 24 months, and then every 6 months during the following years.
A literature search was performed with the following databases: MEDLINE (through PubMed) and Google Scholar. A specific research equation was formulated using the following keywords and/or MeSH terms: “diaphragmatic hernia,” “hepatectomy,” “living donor,” and “liver resection.” In addition, reference lists from eligible studies and relevant review articles were crosschecked to identify additional studies.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement checklist for reporting a systematic review was applied.22 The selection criteria included the following: (i) all types of original articles (including case reports, letters to the editors, and conference abstracts). No time limitation was applied, (ii) adults' patients affected by DH after LR for benign and malignant tumors and in cases of living donation. All studies written in English were retrieved and checked for eligibility. All studies reporting DH after liver-related non-surgical procedures and pediatric diaphragmatic hernias were considered ineligible. All studies that met the inclusion criteria were retrieved and included in this systematic review.
During the study period, the incidence of DH following right-sided major LR was 2.3% (3/131). There were 2 women, 1 man, and the median age was 55 years (43-65 years). All patients underwent open right hepatectomy. Two patients underwent LR for hepatocellular carcinoma and 1 patient underwent LR for mucinous cystadenoma. None of these patients required diaphragm resection. One patient had a 2.2 cm tumor, located in the deep aspect of the right liver, and 2 patients were operated for huge tumors (≥10 cm in diameter) located on the right liver in the vicinity of the right diaphragm. The median tumor size was 9 cm (2.2-15 cm). Neither diaphragm injury nor opening was observed peroperatively. The LR postoperative course was uneventful (Table 1).
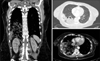
DH was diagnosed in 3 (2.3%) patients with a median delay of 14 months (4-31 months), from the hepatectomy (Fig. 1). One patient underwent emergency laparotomy for bowel obstruction and 2 patients underwent elective DH repair, using an open approach (Fig. 2). As for the diaphragm repair technique, an interrupted suture without mesh was performed in 2 cases and a composite prosthetic mesh (Symbotex™ Composite Mesh, Covidien, USA) was used in 1 case. At last follow-up (1, 5, and 10 months), no recurrence was seen upon cross imaging.
The selected studies included 6 case reports,6911141617 4 retrospective case series,47812 2 letters to the editor,1015 and 2 original articles1318 (Table 2).46789101112131415161718 Overall, 28 cases of DH after LR have been described (excluding our 3 cases). The most common indication of LR were as follows: living donor liver procurement in 11 cases (39.4%), liver metastases in 6 cases (21.4%), HCC in 2 cases (7.1%), adenoma in 2 cases (7.1%), focal nodular hyperplasia in one case (3.6%), and others in 6 cases (21.4%). In the case of living donor liver procurement (11/28), all but one patient underwent right hepatectomy. In the setting of LR for tumors (17/28), open right hepatectomy was performed in 10 cases, laparoscopic left hepatectomy in 3 cases (10.7%), and open left hepatectomy in 1 case. The procedure was not mentioned for 3 cases.18
The mean time between LR and DH was 19 months (1-72 months). The most frequent symptoms were as follows: abdominal pain (50%; 14/28), respiratory symptoms (14.3%; 4/28), and abdominal pain plus respiratory symptoms (14.3%; 4/28). Three (10.7%) patients were asymptomatic. The most commonly herniated organ was the colon (42.8%; 12/28). The hernia repair was performed electively in 46.4% of the cases and emergently in 42.9% of the cases.
Open, laparoscopic, and thoracotomy approaches were used in 67.9% (19/28), 3.5% (1/28), and 17.8% (5/28) of patients, respectively. Data regarding DH repair was not available for 3 cases.
Digestive resection was performed in 8 (28.5%) cases including small bowel resection in 3 cases (10.7%), colon resection in 3 cases (10.7%), appendix resection in 1 case (3.6%), and gastric resection in 1 case (3.6%). As for the diaphragm repair technique, an interrupted suture without mesh was performed in 71.4% (20/28) of the cases. In 17.8% (5/28) of the cases, DH was repaired using a mesh. Of these 5 patients, 2 patients (7.1%) had a non-absorbable mesh. In the remaining 3 cases, data was not available regarding the type of mesh applied. One patient experienced a DH recurrence, which was treated with a mesh using a thoracic approach.
The aim of this study was to provide an overview of the incidence as well as to suggest potential mechanisms of DH after right-sided major LR. Two results should be emphasized.
First, in our experience, the incidence of postoperative DH following right-sided major LR was 2.3%. Second, DH may occur even after right hepatectomy, which does not require diaphragm resection or opening, and in patients with uneventful postoperative course.
This study confirmed that the incidence of this complication is low (2.3%), which is consistent with that of the literature in the setting of LR for tumors (1.1%-6.2%) and in living donor hepatectomy (0.6%-2.3%). In our experience, this complication was not associated with mortality. However, in the literature review (Table 1),46789101112131415161718 one patient died 3 months after hepatectomy, secondary to sepsis from a segment of small bowel that perforated into the DH.13 This suggests that even though this complication is rare, it should be repaired surgically, even for asymptomatic patients.
This study showed that DH occurred with a median delay of 14 months. This was earlier than reported in the literature (19 months). Despite the screening protocol including computed tomography scans every 3 months during the first 24 months, and every 6 months for the following years, the diagnosis of the diaphragmatic hernia was delayed. In three cases, the hernia was evident on imaging, only at that specific time.
In the case of diaphragmatic repair, several surgical techniques are available using an abdominal or thoracic approach. Abdominal DH repair without mesh represents the most commonly used technique reported in the literature (71.4%; 20/28). In the case of DH repair with a mesh, a polypropylene, composite, or biological mesh can be used. The former represents the most commonly used materials. The latter, although more expensive, is preferred due to their low infection rates. Further studies are needed to validate this data.23
Potential intraoperative risk factors of DH following hepatic resection for liver tumors, such as diaphragm resection or opening have been reported.4 Large tumors have not been reported as risk factor by themselves. In a study by Tabrizian et al., half of the patients who had DH were previously operated for huge tumors (≥10 cm).4 This may be explained by the fact that due to the increased intra-abdominal pressure, large tumors are likely to weaken the diaphragm musculature, may predispose patients to DH as the diaphragm is thin and extensively dissected during liver mobilization. Another potential risk factor may be the cautery-related thermal injury to the diaphragm. Electrocautery is routinely used to mobilize the liver from the diaphragm. The routine use of new devices such as bipolar irrigated sealer (Aquamantys®) on hemostasis may increase cautery-related diaphragm injuries.18 In our experience, this bipolar irrigated sealer was used for hemostasis of the diaphragmatic attachments of the right triangular ligament in the last 2 cases. However, studies with more patients are needed to confirm this assumption.
Abdominal (laparoscopic or laparotomy), thoracic, or a combined approach may be used, depending upon the preference of the surgeon, the anatomic location of the defect, and the degree of infra-diaphragmatic adhesions. A thoracic approach might be easier to treat recurrent diaphragmatic hernia, following previous abdominal repair.
In conclusion, despite the increasing technical complexity of LR and new surgical devices used for hemostasis, DH remains a rare complication, following right-sided major resection for liver tumors with an incidence of less than 3%. Tumor burden (size and diaphragm invasion) and technical difficulties (severe adhesion of the right diaphragm, extensive mobilization of the right liver, diaphragm resection, opening or injury) might represent potential causes for DH. Also, thermal surgical devices should be used with caution, when performing right liver mobilization and hemostasis of the diaphragm. A careful follow-up including thoracic and abdominal computed tomography every 3 months, during the first 24 months is advised.
Figures and Tables
 | Fig. 2Intraoperative view of the diaphragmatic hernia. (A) A white arrow shows the right colon flexure incarcerated in the hernia. (B) A white star shows the site of the hernia. (C) Primary closure of the hernia. |
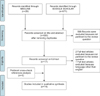 | Fig. 3Flow diagram showing the selection of studies reporting diaphragmatic hernia, following liver resection for systematic review. |
References
1. Lee MK 4th, Gao F, Strasberg SM. Perceived complexity of various liver resections: results of a survey of experts with development of a complexity score and classification. J Am Coll Surg. 2015; 220:64–69.
2. Zimmitti G, Roses RE, Andreou A, Shindoh J, Curley SA, Aloia TA, et al. Greater complexity of liver surgery is not associated with an increased incidence of liver-related complications except for bile leak: an experience with 2,628 consecutive resections. J Gastrointest Surg. 2013; 17:57–64. discussion p.64-65.
3. Rubikas R. Diaphragmatic injuries. Eur J Cardiothorac Surg. 2001; 20:53–57.
4. Tabrizian P, Jibara G, Shrager B, Elsabbagh AM, Roayaie S, Schwartz ME. Diaphragmatic hernia after hepatic resection: case series at a single Western institution. J Gastrointest Surg. 2012; 16:1910–1914.
5. Willemse P, Schütte PR, Plaisier PW. Thoracoscopic repair of a Bochdalek hernia in an adult. Surg Endosc. 2003; 17:162.
6. Hawxby AM, Mason DP, Klein AS. Diaphragmatic hernia after right donor and hepatectomy: a rare donor complication of partial hepatectomy for transplantation. Hepatobiliary Pancreat Dis Int. 2006; 5:459–461.
7. Kousoulas L, Becker T, Richter N, Emmanouilidis N, Schrem H, Barg-Hock H, et al. Living donor liver transplantation: effect of the type of liver graft donation on donor mortality and morbidity. Transpl Int. 2011; 24:251–258.
8. Dieter RA Jr, Spitz J, Kuzycz G. Incarcerated diaphragmatic hernia with intrathoracic bowel obstruction after right liver donation. Int Surg. 2011; 96:239–244.
9. Vernadakis S, Paul A, Kykalos S, Fouzas I, Kaiser GM, Sotiropoulos GC. Incarcerated diaphragmatic hernia after right hepatectomy for living donor liver transplantation: case report of an extremely rare late donor complication. Transplant Proc. 2012; 44:2770–2772.
10. Mizuno S, Tanemura A, Isaji S. Incarcerated left diaphragmatic hernia following left hepatectomy for living donor liver transplantation. Transpl Int. 2014; 27:e65–e67.
11. Jeng KS, Huang CC, Lin CK, Lin CC, Wu JM, Chen KH, et al. Early incarcerated diaphragmatic hernia following right donor hepatectomy: a case report. Transplant Proc. 2015; 47:815–816.
12. Livingstone SM, Andres A, Shapiro AM, Kneteman NN, Bigam DL. Diaphragmatic hernia after living donor right hepatectomy: proposal for a screening protocol. Transplant Direct. 2016; 2:e84.
13. Hemming AW, Reed AI, Langham MR, Fujita S, van der Werf WJ, Howard RJ. Hepatic vein reconstruction for resection of hepatic tumors. Ann Surg. 2002; 235:850–858.
14. Sugita M, Nagahori K, Kudo T, Yamanaka K, Obi Y, Shizawa R, et al. Diaphragmatic hernia resulting from injury during microwave-assisted laparoscopic hepatectomy. Surg Endosc. 2003; 17:1849–1850.
15. Matz D, Kirchhoff P, Kocher TM, Heizmann O. Consecutive cecum perforation due to incarcerated diaphragmatic hernia after liver surgery. Int J Colorectal Dis. 2009; 24:1353–1354.
16. Schellhaas E, Döbler O, Kroesen AJ, Buhr HJ, Hotz HG. Gangrenous intrathoracic appendicitis, a rare cause of right-sided chest pain: report of a case. Surg Today. 2010; 40:874–877.
17. Lodhia JV, Appiah S, Tcherveniakov P, Krysiak P. Diaphragmatic hernia masquerading as a pulmonary metastasis. Ann R Coll Surg Engl. 2015; 97:e27–e29.
18. Patrizi A, Jezequel C, Sulpice L, Meunier B, Rayar M, Boudjema K. Disposable bipolar irrigated sealer (Aquamantys(®)) for liver resection: use with caution. Updates Surg. 2016; 68:171–177.
19. Lim C, Compagnon P, Sebagh M, Salloum C, Calderaro J, Luciani A, et al. Hepatectomy for hepatocellular carcinoma larger than 10 cm: preoperative risk stratification to prevent futile surgery. HPB (Oxford). 2015; 17:611–623.
20. Memeo R, de'Angelis N, Compagnon P, Salloum C, Cherqui D, Laurent A, et al. Laparoscopic vs. open liver resection for hepatocellular carcinoma of cirrhotic liver: a case-control study. World J Surg. 2014; 38:2919–2926.
21. Azoulay D, Marin-Hargreaves G, Castaing D, Adam R, Savier E, Bismuth H. The anterior approach: the right way for right massive hepatectomy. J Am Coll Surg. 2001; 192:412–417.
22. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009; 3:e123–e130.
23. Al-Nouri O, Hartman B, Freedman R, Thomas C, Esposito T. Diaphragmatic rupture: Is management with biological mesh feasible? Int J Surg Case Rep. 2012; 3:349–353.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download