Abstract
Backgrounds/Aims
Mycophenolate mofetil (MMF) has wide inter- and intra-individual variability of mycophenolic acid (MPA) after liver transplantation (LT). On this study, we aimed to analyse the intra-individual variability of MPA concentration in stable adult LT recipients receiving MMF monotherapy and develop a method to determine the target level in the situation of wide intra-individual variability.
Methods
This retrospective cross-sectional study included 30 LT recipients. All patients received MMF monotherapy at a dose of 500 mg twice daily for ≥2 years and were divided into two groups based on renal function. MPA concentration-associated values were presented as mean with standard deviation and coefficient of variation (CV).
Results
The normal renal function group (n=15) showed a mean 12-hour MPA concentration of 2.5±0.5 µg/ml (range, 1.8±0.5 to 3.6±0.7 µg/ml) and a mean CV of 20.4±7.7% (range, 8.7% to 39.4%). In the renal dysfunction group (n=15), the 12-hour MPA concentration fluctuated more widely with a mean value of 3.7±0.9 µg/ml (range, 2.8±0.8 to 5.1±1.2 µg/ml) and a mean CV of 24.5±4.9% (range, 17.1% to 37.5%). The 12-hour MPA concentration was significantly higher in the renal dysfunction group, as compared to the normal renal function group (p=0.001); whereas, the CV was not significantly different between the two groups (p=0.093).
Conclusions
We determined the inter- and intra-individual variability of 12-hour MPA concentration after LT. The results suggested that therapeutic drug monitoring of MPA is necessary due to the inter-individual and intra-individual variability of MMF pharmacokinetics, especially in LT recipients with renal dysfunction.
Mycophenolate mofetil (MMF) is commonly administered after liver transplantation (LT) because of the lack of nephrotoxic effects unlike the calcineurin inhibitor (CNI).123 Mycophenolic acid (MPA), the pharmacokinetically active product of MMF, has potent inhibitory effects on lymphocyte proliferation. MPA is taken up by the liver, where it is glucuronidated to form an inactive compound. MPA metabolites are primarily eliminated by the kidneys.45 Thus, the blood level and clearance of MPA is greatly influenced by renal function.
MMF has been regarded as a categorically-dosed drug probably due to the wide inter- and intra-individual variability of the blood concentration as well as lack of information on clinician-oriented pharmacokinetics.6 Few clinical studies on the relationship between potency and drug concentration have been conducted previously. As a result, MMF has been administered on the basis of personal or institutional experience, without generalized guidelines to perform therapeutic drug monitoring (TDM) for dosage adjustment. Moreover, the wide intra-individual variability of MPA level has been ignored in the clinical setting due to lack of specific knowledge.
Decline in renal function results in significant impairment of the clearance of MPA metabolites, with the consequent increase in blood concentrations of MPA metabolites and augmented immunosuppression.7 Thus, in patients with renal dysfunction, special attention to the MPA pharmacokinetics is required on administration of MMF as the main immunosuppressant. We previously reported that it is necessary to adjust the MMF dosage on an individualized basis based on the results of MPA TDM, particularly for patients with markedly impaired renal function. Based on our experience, patients with renal dysfunction often showed a wide range of variability in the trough MPA concentration; thus, analysis of the pattern of variability in MPA concentration is required.
The primary goal of the present study was to analyse the intra-individual variability of MPA concentration in stable adult LT recipients receiving MMF monotherapy; and the secondary goal was to develop a method to determine the target TDM level in the situation of wide intra-individual variability.
This study was performed as a retrospective cross-sectional study with two arms according to the patients' renal function. Patients who underwent LT operation during the period between January 2003 and December 2011 were included in the study. This cross-sectional study was performed during a 6-month study period from July 2016 to December 2016. The study was approved by the Institutional Review Board of Asan Medical Center (2015-0726).
From our institutional LT database of over 5000 patients, 30 adult LT recipients were selected for inclusion in the study. All selected patents were stable in liver function and had been treated with MMF monotherapy at a daily dose of 1000 mg (500 mg twice per day) for ≥2 years, primarily because they were not able to tolerate CNI-associated nephrotoxicity. Patients who showed any type of graft dysfunction during the study period were excluded from study.
Three MMF agents, Celcept (Roche) and two generic drugs Myrept (Chong Kun Dang pharmaceuticals, Korea) and Myconol (Hanmi pharmaceuticals, Korea), are available in our institution and selection was made based on the physicians' preference. In this study, the bioavailability of these 3 agents was regarded as the same. Myrept was preferentially administered to LT recipients undergoing MMF monotherapy; thus, a half of the study patients received 250 mg or 500 mg tablets of Myrept. Patients receiving different doses of MMF in the morning and evening (e.g., 500 mg plus 750 mg per day) as well as daily administration of MMF ≥1500 mg were excluded from the analysis of 12-hour MPA TDM. Most of the study patients were in the ≥5 years post-LT, hence, the target MPA level was set at 2-3 ng/ml.
In addition, we selected the patients based on serum creatinine levels as the normal renal function group (normal group: mean serum creatinine ≤1.4 mg/dl; n=15) and the renal dysfunction group (dysfunction group: mean serum creatinine 2.0-2.9 mg/dl; n=15). The clinical profiles of patients in these two study groups were adjusted by propensity score matching. None of the patients in the renal dysfunction group underwent hemodialysis during the cross-sectional study period.
The detailed MMF immunosuppression profiles have been described previously.68 Pre-dose 12-hour blood samples were subjected to MPA TDM using an enzyme multiplied immunoassay technique (EMIT: Dade-Behring, Marburg, Germany) performed on a Cobas Mira analyser (Roche, Basel, Switzerland). The results of MPA TDM were usually reported within 2 hours after blood sampling on an outpatient basis.
To avoid bias from intra-individual variations in serum creatinine level, the mean values of consecutive three measurements (usually during 6 months) were used to classify the patients based on renal function. The serial 12-hour MPA levels during the latest 12 months were obtained. Considering that the patients usually visited the outpatient clinic every 2-3 months and MPA TDM was performed at every visit, the MPA TDM sample number per patient was ≥5. MPA TDM-associated values were reported as mean with standard deviation (SD) and coefficient of variation (CV); wherein the CV is the ratio of the SD in trough blood concentration to the mean trough concentration (expressed as percentage) as follows: “CV=SD/Mean×100%”.910
Numerical variables were presented as means with SD and ranges. Continuous variables were compared with the Student t-test. A p-value <0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using SPSS (version 22; IBM, New York, NY) and Statistica (version 6.0; StatSoft, Tulsa, OK) software.
The primary causes of LT in the 30 study patients were hepatitis B virus-associated liver cirrhosis (n=22), alcoholic liver diseases (n=3), acute liver failure (n=3), hepatitis C virus-associated liver cirrhosis (n=1) and primary sclerosing cholangitis (n=1). Among these, there were 25 male patients. The mean patient age was 52.3±7.3 years (range, 34-62). Six patients underwent deceased-donor LT, and 24 patients (84.6%) underwent living-donor LT. There was no case of retransplantation. All patients had been treated with either CNI-based or CNI-MMF combination immunosuppressive therapy early after LT, and gradually converted to MMF monotherapy primarily due to intractable nephrotoxicity. All patients were maintained on a fixed daily dose of MMF (500 mg twice per day) for >2 years recently, during which period, none of the patients experienced significantly noticeable changes in graft function as well as any episode suspected of acute rejection. These clinical profiles were similar in the two study groups after selection with propensity score matching on the primary disease (p=0.87), patient age at LT (p=0.67) and type of LT (p=0.74).
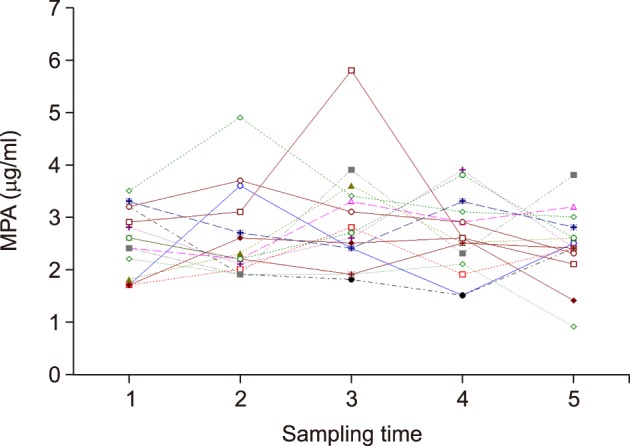
In the normal renal function group (n=15), the 12-hour MPA concentration showed wide fluctuation periodically in most of the patients, probably due to irregular interval after drug ingestion and influence of diet and other medications. Only a few patients showed small fluctuation in 12-hour MPA concentration (Fig. 1). The 12-month mean value with SD of 12-hour MPA concentration in each patient ranged from 1.8±0.5 µg/ml to 3.6±0.7 µg/ml at the mean serum creatinine level of ≤1.4 mg/dl. The mean CV was 20.4%, and ranged from 8.7% to 39.4% in each patient. Overall, the estimated mean 12-hour MPA concentration value was 2.5±0.5 µg/ml and the mean CV value was 20.4±7.7%.
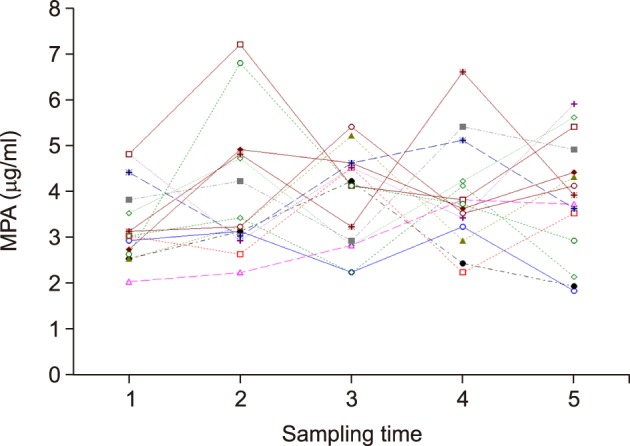
The 12-hour MPA concentration showed more fluctuation periodically in nearly all of patients in the renal dysfunction group (n=15), as compared to the normal renal function group (Fig. 2). The 12-month mean value with SD of 12-hour MPA concentration in each patient ranged from 2.8±0.8 µg/ml to 5.1±1.2 µg/ml at the mean serum creatinine level of between 2.0 mg/dl and 2.9 mg/dl. The CV of each patient was between 17.1% and 37.5%, with a mean CV value of 24.5%. Thus, the overall mean values were estimated at 3.7±0.9 µg/ml of 12-hour MPA concentration and 24.5±4.9% of CV value.
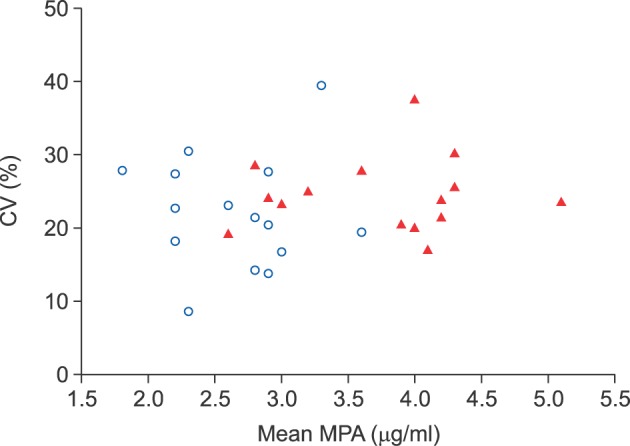
The 12-hour MPA concentrations in the normal and renal dysfunction groups were compared in terms of the mean value (p=0.001) and CV (p=0.093) (Fig. 3).
The 12-hour MPA concentration is widely variable within a patient as well as between patients after LT. Such wide inter- and intra-individual pharmacokinetic variability results in the need for categorical dosing of the immunosuppressant, MMF.5711 However, accumulated experience in clinical administration in LT recipients has facilitated clinicians' attempts to overcome such pharmacokinetic variability through evidence-based analysis.
In the early period of MMF use, high doses of MMF of up to 1000-3000 mg/day were recommend even in the situation of concurrent use of CNI in the western countries, which might be an overestimated dosage to compensate for poor absorption of MMF. High dosages might be acceptable in a considerable proportion of patients; however, empirical MMF administration occasionally resulted in unexpected infectious episodes.6 In our experience of MMF administration in >1000 LT patients, we have rarely administered MMF 1500 mg per day after the early posttransplant period. In everyday practice, we have frequently performed MPA TDM especially during the early period after MMF administration in order to screen out the poor MMF absorbers. Recently, for poor MMF absorbers, MMF can be replaced with mammalian target of rapamycin (mTOR) inhibitors, despite other serious adverse side-effects.1213 In the Korean setting, frequent performance of MPA TDM after LT is fully covered by the nationwide social medical insurance, thus, it is possible to perform MPA TDM in all cases.
The effective therapeutic dose of MPA is currently unknown. We previously reported a relatively low inter-individual correlation between MMF dosage and 12-hour MPA concentration (r2=0.271, R=0.521, p<0.001); based on the posttransplant period, r2 was 0.153 at 0-3 months, 0.228 at 4-12 months, 0.508 at 1-2 years, 0.293 at 3-5 years, and 0.247 at >5 years. Regarding 12-hour tacrolimus concentration, a similar degree of inter-individual variation was observed (r2=0.247, R=0.497, p<0.001); based on the posttransplant period, r2 was 0.056 at 0-3 months, 0.162 at 4-12 months, 0.085 at 1-2 years, 0.071 at 3-5 years, and 0.213 at >5 years. These results suggest that the inter-individual drug-concentration variation of tacrolimus was comparable to that of MMF.6 Therefore, administration of MMF as MMF-based immunosuppression is not recommended in cases wherein MPA TDM is not conducted.
Inter-individual variation of MPA concentration is the most important point to consider before intentional conversion to MMF monotherapy. In our LT program, nearly all of the patients who received MMF monotherapy were “naturally selected” through a process of trial-and-error, such that most of the poor absorbers dropped out. In practice, we have not attempted MMF monotherapy if the 12-hour MPA trough level was <1.0 µg/ml after daily intake of 1500 mg MMF because such cases can be regarded as poor MMF absorbers. Any compulsory application of MMF monotherapy without consideration of MPA TDM might result in higher rates of acute rejection, which were presented in literature.1415161718 In contrast, a prospective randomised trial of MMF monotherapy did not present any episodes of acute rejection during 5 years of the follow-up study period.19
In this study, the primary study goal was focused on the intra-individual variability of MPA concentration. In patients with normal renal function, the mean of individual CV value of 12-hour MPA concentration was 2.5±0.5 µg/ml with 20.4±7.7% of CV value, which was lower than expected. In studies with renal transplantation, high inter-individual variability of tacrolimus was reportedly associated with a higher incidence of acute rejection.910 In contrast, in patients with renal dysfunction, the mean of individual CV value of 12-hour MPA concentration was 3.7±0.9 µg/ml of 12-hour MPA concentration with 24.5±4.9% of CV value, suggesting that the 12-hour MPA concentration significantly increased as a result of renal dysfunction; however, the intra-individual variability was not significantly influenced. Thus, the general concept of MMF dose-adjustment based on MPA TDM can be applicable to patients with renal dysfunction. Based on the practical need, our secondary study goal was to develop a method to determine the target MPA level in the situation of intra-individual variability in patients with renal dysfunction.
Pharmacokinetically, MPA metabolites are eliminated primarily via the kidneys, thus MPA concentration is greatly affected by renal function.45 The results of this study demonstrated that noticeable increase in serum creatinine levels significantly raised the MPA level. We previously demonstrated that patients administering 500 mg MMF twice per day (MMF 1000 mg group) showed a 12-hour MPA trough level of 1.20±0.35 g/ml at the serum creatinine level of ≤1.4 mg/dl, 2.78±1.19 g/ml at the serum creatinine level of 1.56-3.35 mg/dl without hemodialysis, and 3.83±0.87 g/ml at higher serum creatinine level of 3.55-8.31 mg/dl with hemodialysis.8 Thus, the importance of meticulous MPA TDM especially in patients with renal dysfunction is emphasized.
The present study had several limitations. First, this is a retrospective, single-center study with a small number of patients. Thus, multi-center studies are necessary to recruit more patients. Second, we selected only the patients showing stable liver function on MMF monotherapy, thus they might be highly selected already. Studies are required that include a patient pool that reflects various clinical situations in actual practice.
In conclusion, the results of this study provided the detailed information on inter- and intra-individual variability of 12-hour MPA concentration. MPA TDM is necessary to reasonably cope with the inter-individual and intra-individual variability of MMF pharmacokinetics in LT recipients.
ACKNOWLEDGEMENTS
This study was financially supported by the Organ Transplantation Center of Asan Medical Center and Chong Kun Dang pharmaceutical Corp. (University of Ulsan cooperative system fund 2015-1518).
References
1. Jain A, Vekatramanan R, Eghtesad B, Gadomski M, Mohanka R, Marcos A, et al. Long-term outcome of adding mycophenolate mofetil to tacrolimus for nephrotoxicity following liver transplantation. Transplantation. 2005; 80:859–864. PMID: 16210976.

2. Jain A, Kashyap R, Dodson F, Kramer D, Hamad I, Khan A, et al. A prospective randomized trial of tacrolimus and prednisone versus tacrolimus, prednisone and mycophenolate mofetil in primary adult liver transplantation: a single center report. Transplantation. 2001; 72:1091–1097. PMID: 11579306.
3. Perry I, Neuberger J. Immunosuppression: towards a logical approach in liver transplantation. Clin Exp Immunol. 2005; 139:2–10. PMID: 15606606.

4. Sintchak MD, Fleming MA, Futer O, Raybuck SA, Chambers SP, Caron PR, et al. Structure and mechanism of inosine monophosphate dehydrogenase in complex with the immunosuppressant mycophenolic acid. Cell. 1996; 85:921–930. PMID: 8681386.

5. Brunet M, Cirera I, Martorell J, Vidal E, Millán O, Jiménez O, et al. Sequential determination of pharmacokinetics and pharmacodynamics of mycophenolic acid in liver transplant patients treated with mycophenolate mofetil. Transplantation. 2006; 81:541–546. PMID: 16495801.

6. Hwang S, Lee SG, Ahn CS, Kim KH, Moon DB, Ha TY, et al. A clinical assessment of mycophenolate drug monitoring after liver transplantation. Clin Transplant. 2010; 24:E35–E42. PMID: 20070319.

7. Jain A, Venkataramanan R, Hamad IS, Zuckerman S, Zhang S, Lever J, et al. Pharmacokinetics of mycophenolic acid after mycophenolate mofetil administration in liver transplant patients treated with tacrolimus. J Clin Pharmacol. 2001; 41:268–276. PMID: 11269567.

8. Park YH, Hwang S, Song GW, Jung DH, Ahn CS, Kim KH, et al. Correlation between mycophenolic acid blood level and renal dysfunction in stable liver transplant recipients receiving mycophenolate monotherapy. Transplant Proc. 2014; 46:811–815. PMID: 24767354.

9. Borra LC, Roodnat JI, Kal JA, Mathot RA, Weimar W, van Gelder T. High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol Dial Transplant. 2010; 25:2757–2763. PMID: 20190242.

10. Shuker N, van Gelder T, Hesselink DA. Intra-patient variability in tacrolimus exposure: causes, consequences for clinical management. Transplant Rev (Orlando). 2015; 29:78–84. PMID: 25687818.

11. Tredger JM, Brown NW, Adams J, Gonde CE, Dhawan A, Rela M, et al. Monitoring mycophenolate in liver transplant recipients: toward a therapeutic range. Liver Transpl. 2004; 10:492–502. PMID: 15048791.

12. Glover TE, Watson CJ, Gibbs P, Bradley JA, Ntzani EE, Kosmoliaptsis V. Conversion from calcineurin to mammalian target of rapamycin inhibitors in liver transplantation: a meta-analysis of randomized controlled trials. Transplantation. 2016; 100:621–629. PMID: 26636736.
13. Hüsing A, Schmidt M, Beckebaum S, Cicinnati VR, Koch R, Thölking G, et al. Long-term renal function in liver transplant recipients after conversion from calcineurin inhibitors to mTOR inhibitors. Ann Transplant. 2015; 20:707–713. PMID: 26608590.

14. Bilbao I, Castells L, Rojas L, Cancino J, Dopazo C, Castro E, et al. Immunosuppression based on mycophenolate mofetil in stable liver transplanted patients. Int Immunopharmacol. 2006; 6:1977–1983. PMID: 17161351.

15. Orlando G, Baiocchi L, Cardillo A, Iaria G, De Liguori Carino N, De Luca L, et al. Switch to 1.5 grams MMF monotherapy for CNI-related toxicity in liver transplantation is safe and improves renal function, dyslipidemia, and hypertension. Liver Transpl. 2007; 13:46–54. PMID: 17154392.

16. Schmeding M, Neumann UP, Neuhaus R, Neuhaus P. Mycophenolate mofetil in liver transplantation--is monotherapy safe? Clin Transplant. 2006; 20(Suppl 17):75–79. PMID: 17100705.
17. Fairbanks KD, Thuluvath PJ. Mycophenolate mofetil monotherapy in liver transplant recipients: a single center experience. Liver Transpl. 2004; 10:1189–1194. PMID: 15350013.

18. Reich DJ, Clavien PA, Hodge EE. MMF Renal Dysfunction after Liver Transplantation Working Group. Mycophenolate mofetil for renal dysfunction in liver transplant recipients on cyclosporine or tacrolimus: randomized, prospective, multicenter pilot study results. Transplantation. 2005; 80:18–25. PMID: 16003228.

19. Schmeding M, Kiessling A, Neuhaus R, Heidenhain C, Bahra M, Neuhaus P, et al. Mycophenolate mofetil monotherapy in liver transplantation: 5-year follow-up of a prospective randomized trial. Transplantation. 2011; 92:923–929. PMID: 21832958.

Fig. 1
Serial measurement of 12-hour mycophenolic acid (MPA) concentration in liver transplant recipients with normal renal function after administration of mycophenolate mofetil 500 mg twice per day.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download