Abstract
Objective
Measurement of the degree of stenosis is not enough to decide on the treatment strategy for patients with carotid stenosis. Plaque morphology examination is needed for such a decision-making. Thus, we evaluated the usefulness of plaque magnetic resonance imaging (MRI) to decide on the modality of treatment for patients with carotid atherosclerotic plaques.
Materials and Methods
Fifteen patients presenting with carotid stenosis between 2014 and 2016 were included. They underwent angiography for measurement of the degree of stenosis. Carotid plaques were visualized using MRI.
Results
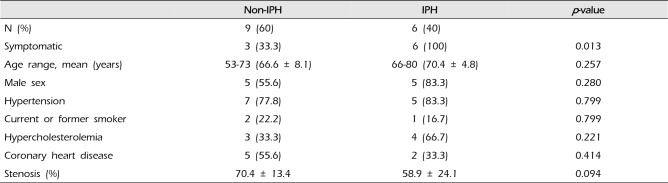
There were six (40%) stable and nine (60%) unstable plaques. Seven symptomatic patients (77.7%) had unstable lesions and two symptomatic patients (33.3%) had stable lesions (p = 0.096). There were six (40%) intraplaque hemorrhage (IPH) cases. There were six symptomatic patients (100%) in the IPH group and three symptomatic patients (33.3%) in the non-IPH group (p = 0.013). The mean stenosis degree was 58.9% in the IPH group and 70.4% in the non-IPH group (p = 0.094). Symptoms occurred irrespective of the degree of the stenosis in the IPH groups. In the IPH group, the recurrent ischemic cerebrovascular event rate was 33.3%. Particularly, the recurrent ischemic cerebrovascular event rate was 66.7% in the IPH group with mild stenosis treated with medications.
Conclusion
IPH in plaque MRI is significantly associated with ischemic symptoms and has a high risk for subsequent ischemic cerebrovascular events irrespective of the degree of stenosis. Plaque MRI is a useful tool in predicting symptomatic risks for carotid stenosis irrespective of the degree of such stenosis.
Ischemic stroke and transient ischemic attack (TIA) are commonly caused by carotid atherosclerosis. Measurement of carotid artery stenosis is a standard method for evaluating the stroke risk. However, Ambrose et al.2) suggested that myocardial infarction (MI) frequently occurs from non-severe coronary artery lesion on coronary angiogram. Thus, they did not estimate MI risk with simple coronary artery stenosis.2) About 90% of conservatively treated patients with carotid artery stenosis did not have a cerebrovascular event for 5 years.13)19) It showed that the evaluation of carotid artery stenosis is not enough to determine on the treatment strategy of patients with carotid atherosclerotic plaques. Histopathological studies of plaque morphology identified specific parameters indicating plaque rupture. Carotid plaques characterized by a thinned fibrous cap with a lipid-rich necrotic core or by intraplaque hemorrhage (IPH) indicate unstable, rupture-prone lesions with a high risk of spontaneous thromboembolic events.3)5)8)10)12)18)
High-resolution magnetic resonance imaging (MRI) is suitable for evaluation of plaque morphology, because it is noninvasive and has excellent capabilities of discriminating tissue characteristics compared with other imaging modalities.11)16)17)20) Therefore, we evaluated the usefulness of plaque MRI to decide on the modality of treatment for patients with carotid atherosclerotic plaques.
Between April 2014 and January 2016, we retrospectively evaluated 15 patients who were treated for carotid stenosis at our stroke center. All patients underwent carotid artery MRI examination and carotid artery angiography.
The patients underwent MRI performed on a 3.0T Achieva Tx (Phillips, Amsterdam, The Netherlands). Carotid plaques were visualized using MRI. T1, T2, time-of-flight (TOF), T1-enhanced, simultaneous non-contrast angiography and intraplaque hemorrhage (SNAP), and magnetization-prepared rapid gradient-echo (MPRAGE) studies were performed. The MRI findings of each plaque component were as follows: the lipid-rich necrotic core showed a hyposignal on the T2-weighted images; calcification showed a hyposignal on the TOF, T1-weighted, and T2-weighted images; hemorrhage showed a hypersignal on the TOF and T1-weighted images; and fibrous tissues showed enhancements on the T1-enhanced image (Table 1). Each lesion was classified using the modified American Heart Association (AHA) classification for MRI.4) Type I-II has near normal wall thickness with no calcification. Type III has diffuse intimal thickening or small eccentric plaques with no calcification. Type IV-V involves a plaque with a lipid or necrotic core surrounded by fibrous tissues with possible calcifications. Type VI involves a complex plaque with a possible surface defect, hemorrhage, or thrombus. Type VII involves a calcified plaque. Type VIII involves a fibrotic plaque without a lipid core and with possible small calcifications. Among them, Types IV-V and VI were classified as unstable plaques, while the others were classified as stable plaques. Further, we classified them according to the presence of IPH.
The mean patient age was 70.2 ± 8.2 (range, 53–80) years. The male-to-female sex ratio was 10:5. Nine patients were symptomatic (60%). There were Four (26.7%) cases of mild stenosis (< 50%), two (13.3%) of moderate stenosis (50–69%), and nine (60%) of severe stenosis (> 70%). Diabetes mellitus (DM) was diagnosed in five patients (33.3%). Hypertension was diagnosed in 12 patients (80%). Hyperlipidemia was diagnosed in seven patients (46.7%). Coronary heart disease was diagnosed in seven patients (46.7%). One patient was lost to follow-up. One patient died shortly due to pneumonia. The mean follow-up duration was 19.7 months (range, 5–33 months). During this period, two patients (13.3%) developed an ipsilateral ischemic cerebrovascular event. Three patients (20%) presented with bilateral stenosis. Nine patients (60%) underwent carotid angioplasty and stenting (CAS). One patient (6.7%) underwent carotid endarterectomy (CEA). Five patients (33.3%) underwent medical treatment.
The lesion types of the carotid plaques were as follows: two lesions were classified under type III (13.3%), three lesions under type IV-V (20%), six lesions under type VI (40%), and four lesions under type VII (26.7%). There were six (40%) stable (types III and VII) and nine (60%) unstable (types IV-V and VI) plaques. There were six (40%) IPH cases (Table 2).
In the stable lesion types, there were one (16.7%) mild stenosis, two (33.3%) moderate stenosis, and three (50%) severe stenosis. The mean stenosis degree was 72.8%. In the unstable lesion types, there were three (33.3%) mild stenosis, no moderate stenosis, and six (66.7%) severe stenosis. The mean stenosis degree was 61.1%. There were seven symptomatic patients (77.7%) with unstable lesions and two symptomatic patients (33.3%) with stable lesions (p = 0.096) (Table 3).
All patients with IPH (n = 6) were symptomatic (100%). There were three symptomatic patients (33.3%) in the non-IPH group (p = 0.013). The mean stenosis degree was 58.9% in the IPH group and 70.4% in the non-IPH group (p = 0.094) (Table 4). In the non-IPH group, the symptomatic patients had severe stenosis. However, the symptoms occurred irrespective of the degree of stenosis in the IPH group.
In the stable lesion types, the patients with mild and moderate stenosis (n = 3, 50%) were treated with medications and those with severe stenosis (n = 3, 50%) with CAS. In the unstable lesion types, three patients with mild stenosis (33.3%) were treated with medications; however, two patients were treated for ischemic cerebrovascular events during the follow-up period (66.7%). Further, the patients with severe stenosis (n = 6, 66.7%) were treated with CAS. In the stable lesion types, none of the patients (0%) presented with ischemic cerebrovascular events during the follow-up period. In the unstable lesion types, two patients (22.2%) had recurrent ischemic cerebrovascular events during the follow-up period. The plaque MRI of these patients revealed IPH. In the IPH group, the recurrent ischemic cerebrovascular event rate was 33.3%. Particularly, the recurrent ischemic cerebrovascular event rate was 66.7% in the IPH group with mild stenosis treated with medications.
A 67-year-old man was admitted due to mild motor weakness of the right upper extremity (grade 4+) and mild dysarthria. He had hypertension and hyperlipidemia. He had a history of cerebral infarction twice, 4 years and 4 months earlier. A month ago, he was treated with medications for a mild left cervical internal carotid artery (ICA) stenosis (Fig. 1A). MRI on admission showed multiple cerebral infarctions at the left cerebral hemisphere (Fig. 1B). The degree of carotid stenosis was not changed (14.7%), however, small ulceration was demonstrated on cerebral angiogram (Fig. 1C). Plaque MRIs showed IPH (Fig. 2). On T1 enhanced image, focal disruption of fibrous cap was demonstrated (Fig. 2D, E). He was treated conservatively. After 8 days, he had a recurrent cerebral infarction (Fig. 3). His motor weakness was aggravated at the right upper extremity (grade 4) and lower extremity (grade 4+). He underwent CEA. In the operative findings, the carotid plaque had an IPH and ulcer as shown in the MRI (Fig. 4).
A 75-year-old man was admitted due to dizziness. He had no DM, hypertension, and hyperlipidemia. He was treated for coronary heart disease 20 years prior. He had no motor weakness. The degree of carotid stenosis was mild (35%) (Fig. 5). Plaque MRIs showed IPH in the right ICA and chronic complete occlusion in the left ICA (Fig. 6). He was treated with medications. After 27 months, he had a recurrent ischemic cerebrovascular event, amaurosis fugax. Cervical angiogram showed progression of carotid stenosis to 80% (Fig. 7). He underwent CAS. Right ICA angiogram showed more dilatation of ICA after CAS (Fig. 8).
High-resolution MRI is ideal for the evaluation of the plaque status. Cai et al. modified the AHA classification of atherosclerotic lesions by MRI.4) Using this modified classification, plaques with a lipid-rich necrotic core surrounded by fibrous tissues with possible calcification can be classified as lesion types IV-V and complex plaques with a possible surface defect, hemorrhage, or thrombus as lesion type VI.6)21) Cai et al. reported that high sensitivity and specificity were achieved from type III to type VII lesions.4) The sensitivity and specificity of MRI in categorizing each lesion type were as follows: type I-II, 67% and 100%; type III, 81% and 98%; type IV-V, 84% and 90%; type VI, 82% and 91%; type VII, 80% and 94%; and type VIII, 56% and 100%, respectively.4)
Lipid-rich necrotic core and IPH demonstrated in atherosclerotic plaques are strongly associated with increased risks of ischemic cerebrovascular events as well as plaque progression. IPH has also been regarded to be a potentially important factor in treatment planning. Altaf et al.1) found MRI-defined vulnerable plaque features, such as IPH, to be related to recurrent cerebral events when evaluating symptomatic patients with mild to moderate carotid artery stenosis. The presence of IPH significantly predicted recurrence of various ischemic event (e.g., stroke, TIA, and amaurosis fugax) with a hazard ratio (HR) of 9.8 (95% confidence interval [CI] 1.3–75.1); all other patient-related factors, including age (HR = 1.0), hypertension (HR = 1.8), ischemic heart disease (HR = 0.4), DM (HR = 1.4), smoking (HR = 1.3), and degree of stenosis (HR = 1.5; 95% CI 0.5–4.9) had lower HRs.1) Esposito-Bauer et al.7) also found that MRI-defined vulnerable plaques are at a high risk for future cerebrovascular events in asymptomatic patients. Only patients harboring with high-risk lesion types IV-V and VI occurred an ipsilateral cerebrovascular event; none of the patients presenting with stable lesion types III, VII, and VIII (11.7% vs. 0%) during the follow-up developed such an event. The carotid plaques of four (44.4%) of these nine patients who suffered an ischemic event during the follow-up were classified as lesion type IV-V; five (55.6%) of these patients presented with lesion type VI. The event-free survival rate was higher among the patients with the MRI-defined stable lesion types (III, VII, and VIII) than among patients with the MRI-defined high-risk lesion types (IV-V and VI) (58-month event-free probability 100% vs. 67.8%; log rank test p < 0.0001).7) Our findings are consistent with such results. In our study, only the patients with unstable lesions (22.2% vs. 0%) presented with ischemic cerebrovascular events (ischemic stroke) during the follow-up period.
Rothwell et al. reported that the benefits of CEA depend upon patient-specific factors other than the degree of stenosis in symptomatic moderate stenosis groups.14) Plaque characters, such as the integrity of the fibrous cap and the size of the lipid core, are important for contributing to the stability of the plaque and are found in both symptomatic and asymptomatic carotid arteries.9)15)
In our study, the patients with mild stenosis suffered recurrent cerebral infarction during the follow-up period. They had IPH on MRI. However, the symptomatic patients in the non-IPH group had severe stenosis. We think that if IPH is present, CAS or CEA should be considered irrespective of the degree of stenosis.
Plaque MRI is a useful tool in predicting symptomatic risks for carotid stenosis irrespective of the degree of such stenosis. IPH in plaque MRI is significantly associated with ischemic symptoms and has a high risk for subsequent ischemic cerebrovascular events irrespective of the degree of stenosis. If IPH is demonstrated in plaque MRI, considering proactive treatment may be reasonable to prevent future ischemic event.
References
1. Altaf N, Daniels L, Morgan PS, Auer D, MacSweeney ST, Moody AR, et al. Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. J Vasc Surg. 2008; 2. 47(2):337–342. PMID: 18164171.

2. Ambrose JA, Tannenbaum MA, Alexopoulos D, Hjemdahl-Monsen CE, Leavy J, Weiss M, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988; 7. 12(1):56–62. PMID: 3379219.

3. Bassiouny HS, Sakaguchi Y, Mikucki SA, McKinsey JF, Piano G, Gewertz BL, et al. Juxtalumenal location of plaque necrosis and neoformation in symptomatic carotid stenosis. J Vasc Surg. 1997; 10. 26(4):585–594. PMID: 9357458.

4. Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002; 9. 106(11):1368–1373. PMID: 12221054.

5. Carr S, Farb A, Pearce WH, Virmani R, Yao JS. Atherosclerotic plaque rupture in symptomatic carotid artery stenosis. J Vasc Surg. 1996; 5. 23(5):755–765. discussion 765-6. PMID: 8667496.

6. Chu B, Kampschulte A, Ferguson MS, Kerwin WS, Yarnykh VL, O'Brien KD, et al. Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke. 2004; 5. 35(5):1079–1084. PMID: 15060318.

7. Esposito-Bauer L, Saam T, Ghodrati I, Pelisek J, Heider P, Bauer M, et al. MRI plaque imaging detects carotid plaques with a high risk for future cerebrovascular events in asymptomatic patients. PLoS One. 2013; 7. 8(7):e67927. PMID: 23894291.

8. Falk E. Why do plaques rupture? Circulation. 1992; 12. 86(6 Suppl):III30–III42. PMID: 1424049.
9. Golledge J, Greenhalgh RM, Davies AH. The symptomatic carotid plaque. Stroke. 2000; 3. 31(3):774–781. PMID: 10700518.

10. Lusby RJ, Ferrell LD, Ehrenfeld W, Stoney R, Wylie EJ. Carotid plaque hemorrhage: its role in production of cerebral ischemia. Arch Surg. 1982; 11. 117(11):1479–1488. PMID: 6182861.
11. Maynor CH, Charles HC, Herfkens RJ, Suddarth SA, Johnson GA. Chemical shift imaging of atherosclerosis at 7.0 Tesla. Invest Radiol. 1989; 1. 24(1):52–60. PMID: 2917823.

12. Mofidi R, Crotty TB, McCarthy P, Sheehan SJ, Mehigan D, Keaveny TV. Association between plaque instability, angiogenesis and symptomatic carotid occlusive disease. Br J Surg. 2001; 7. 88(7):945–950. PMID: 11442525.

13. Robless P, Baxter A, Byrd S, Emson M, Halliday A. Prevalence of asymptomatic CT infarcts in the ongoing Asymptomatic Carotid Surgery Trial (ACST). Int Angiol. 1998; 9. 17(3):194–200. PMID: 9821034.
14. Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003; 1. 361(9352):107–116. PMID: 12531577.

15. Takaya N, Yuan C, Chu B, Saam T, Underhill H, Cai J, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI--initial results. Stroke. 2006; 3. 37(3):818–823. PMID: 16469957.

16. Toussaint JF, LaMuraglia GM, Southern JF, Fuster V, Kantor HL. Magnetic resonance images lipid, fibrous, calcified, hemorrhagic, and thrombotic components of human atherosclerosis in vivo. Circulation. 1996; 9. 94(5):932–938. PMID: 8790028.

17. Toussaint JF, Southern JF, Fuster V, Kantor HL. T2-weighted contrast for NMR characterization of human atherosclerosis. Arterioscler Thromb Vasc Biol. 1995; 10. 15(10):1533–1542. PMID: 7583524.
18. Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005; 10. 25(10):2054–2061. PMID: 16037567.
19. Walker MD, Marler JR, Goldstein M, Grady PA, Toole JF, Baker WH, et al. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995; 5. 273(18):1421–1428. PMID: 7723155.

20. Yuan C, Hatsukami TS, Beach KW, Hayes CE, Nelson JA, Ferguson MS, et al. In vivo MR evaluation of atherosclerosis in human carotid artery with use of phased-array coils. J Vasc Interv Radiol. 1996; Jan-Feb. 7(1):46–48.

21. Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation. 2001; 10. 104(17):2051–2056. PMID: 11673345.

Fig. 1
(A) CT angiography shows mild stenosis of left ICA before cerebral infarction. (B) MRI showing multiple cerebral infarctions at the left cerebral hemisphere. (C) Left CCA angiogram shows no significant change of stenosis degree except small ulceration (black arrow) after recurrent cerebral infarction. CT = computed tomography; ICA = internal carotid artery; MRI = magnetic resonance imaging; CCA = common carotid artery.
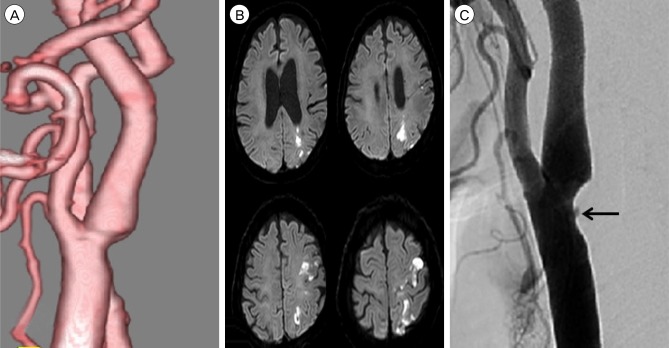
Fig. 2
Plaque MRIs show IPH. IPH (pink arrow) shows high-signal intensity on T1-weighted (A), TOF (B), SNAP (F), MPRAGE (G) images. On T2-weighted image (C), IPH (arrow) shows iso-signal intensity. On T1 enhanced image (D, E), focal disruption of fibrous cap (white arrow) is demonstrated. Focal disruption of fibrous cap in (E) is corresponding to plaque ulcer shown in DSA. MRI = magnetic resonance imaging; IPH = intraplaque hemorrhage; TOF = time-of-flight; SNAP = simultaneous non-contrast angiography and intraplaque hemorrhage; MPRAGE = magnetization-prepared rapid gradient-echo; DSA = digital subtraction angiography.
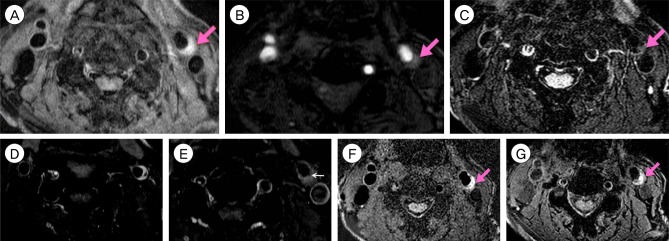
Fig. 3
MRI shows a new cerebral infarction at border zone of left hemisphere. MRI = magnetic resonance imaging.
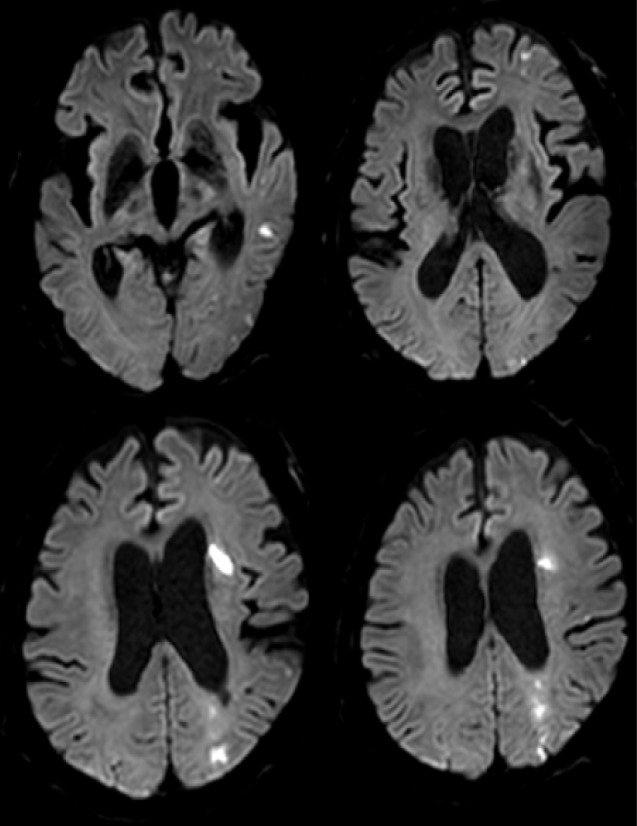
Fig. 4
Operative findings. (A, B) Carotid plaque has IPH and ulcer as shown in the MRI. IPH = intraplaque hemorrhage; MRI = magnetic resonance image.
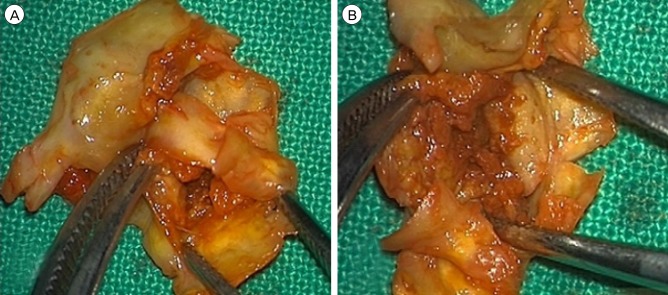
Fig. 5
Right CCA 3D angiogram shows mild stenosis of right ICA. CCA = common carotid artery; ICA = internal carotid artery.
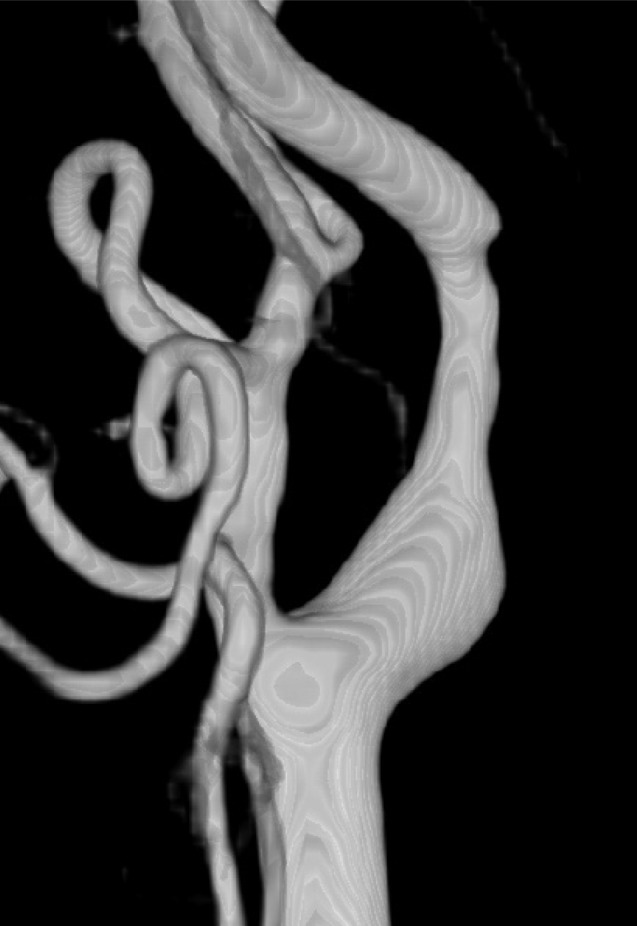
Fig. 6
Plaque MRIs show IPH. IPH (pink arrow) shows high-signal intensity on T1-weighted (A), TOF (B), SNAP (E), MPRAGE (F) images. On T2-weighted image (C), IPH shows high-signal intensity (white arrow). On T1 enhanced images (D), fibrous cap is intact (yellow arrowhead). MRI = magnetic resonance image; IPH = intraplaque hemorrhage; TOF = time-of-flight; SNAP = simultaneous non-contrast angiography and intraplaque hemorrhage; MPRAGE = magnetization-prepared rapid gradient-echo.
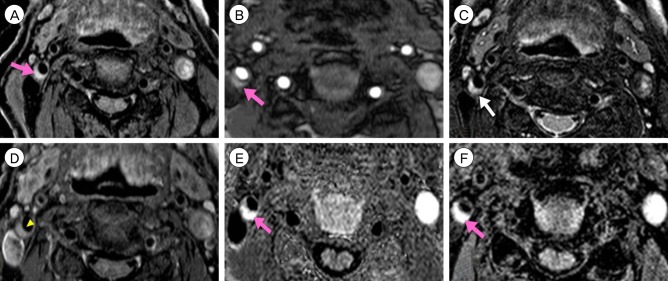
Fig. 7
Right CCA 3D angiogram showing severe focal stenosis of the right proximal cervical ICA. CCA = common carotid artery; ICA = internal carotid artery.
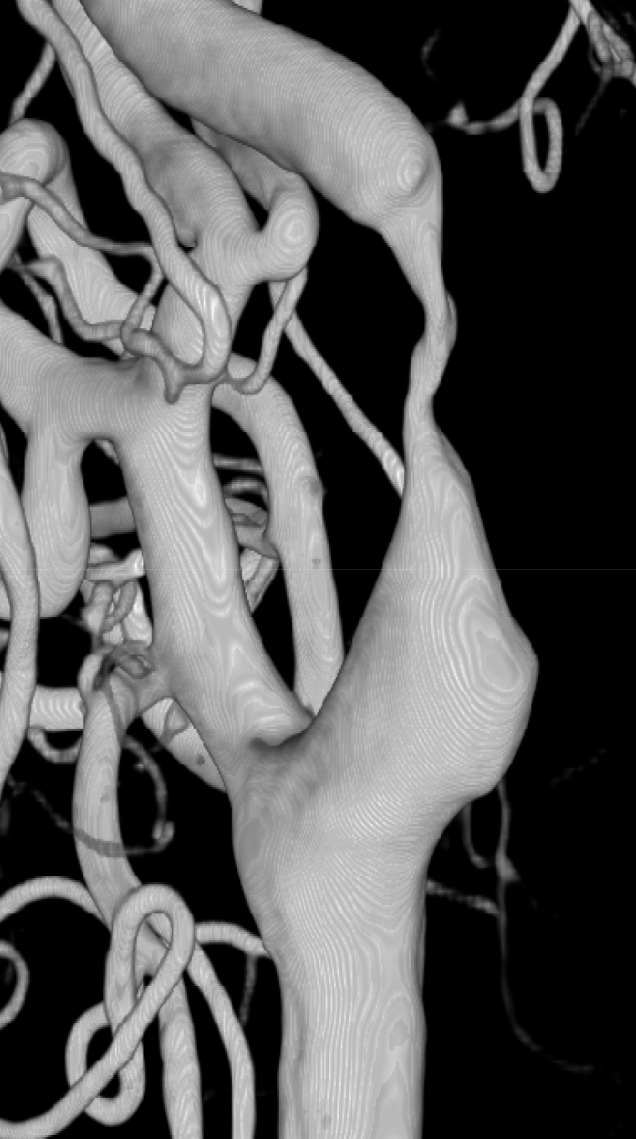
Fig. 8
Right CCA angiogram shows dilatation of ICA after CAS. CCA = common carotid artery; ICA = internal carotid artery; CAS = carotid angioplasty and stenting.
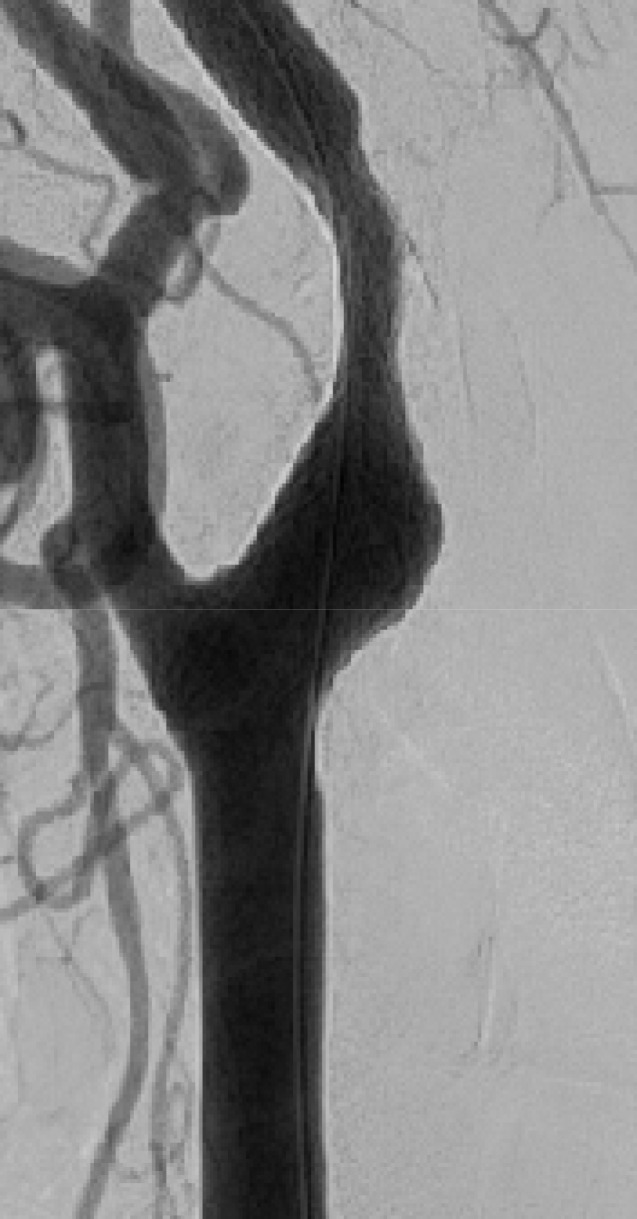
Table 1
MRI findings of the plaque components

| TOF | T1 | T2 | T1-enhanced | |
|---|---|---|---|---|
| Fibrous tissue | Iso/Hypo | Iso | Hyper | + |
| Lipid-rich | Iso | Iso/Hyper | Hypo | - |
| Calcification | Hypo | Hypo | Hypo | - |
| Hemorrhage | Hyper | Hyper | Variable | - |
Table 2
Patient data (carotid plaque status via MRI)
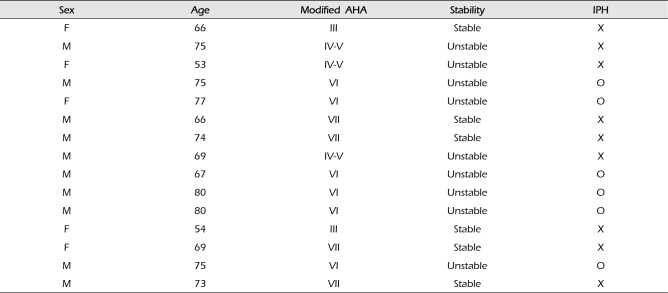
Table 3
Patient data (grouped according to stability)
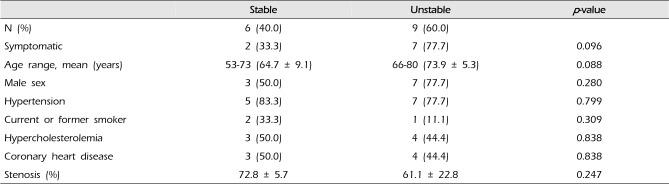
Table 4
Patient data (grouped according to the presence of IPH)




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download