1. Abu-Fadel MS, Sparling JM, Zacharias SJ, Aston CE, Saucedo JF, Schechter E, et al. Fluoroscopy vs. traditional guided femoral arterial access and the use of closure devices: a randomized controlled trial. Catheter Cardiovasc Interv. 2009; 10. 74(4):533–539. PMID:
19626694.

2. Ahn HY, Lee HJ, Lee HJ, Yang JH, Yi JS, Lee IW. Assessment of the optimal site of femoral artery puncture and angiographic anatomical study of the common femoral artery. J Korean Neurosurg Soc. 2014; 8. 56(2):91–97. PMID:
25328644.

3. Altin RS, Flicker S, Naidech HJ. Pseudoaneurysm and arteriovenous fistula after femoral artery catheterization: association with low femoral punctures. AJR Am J Roentgenol. 1989; 3. 152(3):629–631. PMID:
2783816.

4. Ammann P, Brunner-La Rocca HP, Angehrn W, Roelli H, Sagmeister M, Rickli H. Procedural complications following diagnostic coronary angiography are related to the operator's experience and the catheter size. Catheter Cardiovasc Interv. 2003; 5. 59(1):13–18. PMID:
12720234.

5. Chandrasekar B, Doucet S, Bilodeau L, Crepeau J, deGuise P, Gregoire J, et al. Complications of cardiac catheterization in the current era: a single-center experience. Catheter Cardiovasc Interv. 2001; 3. 52(3):289–295. PMID:
11246238.

6. Chinikar M, Ahmadi A, Heidarzadeh A, Sadeghipour P. Imaging or trusting on surface anatomy? A comparison between fluoroscopic guidance and anatomic landmarks for femoral artery access in diagnostic cardiac catheterization. A randomized control trial. Cardiovasc Interv Ther. 2014; 1. 29(1):18–23. PMID:
23959379.

7. Dotter CT, Rösen J, Robinson M. Fluoroscopic guidance in femoral artery puncture. Radiology. 1978; 4. 127(1):266–267. PMID:
635197.

8. Ellis SG, Bhatt D, Kapadia S, Lee D, Yen M, Whitlow PL. Correlates and outcomes of retroperitoneal hemorrhage complicating percutaneous coronary intervention. Catheter Cardiovasc Interv. 2006; 4. 67(4):541–545. PMID:
16547938.

9. Farouque HO, Tremmel JA, Raissi Shabari F, Aggarwal M, Fearon WF, Ng MK, et al. Risk factors for the development of retroperitoneal hematoma after percutaneous coronary intervention in the era of glycoprotein IIb/IIIa inhibitors and vascular closure devices. J Am Coll Cardiol. 2005; 2. 45(3):363–368. PMID:
15680713.

10. Gedikoglu M, Oguzkurt L, Gur S, Andic C, Sariturk C, Ozkan U. Comparison of ultrasound guidance with the traditional palpation and fluoroscopy method for the common femoral artery puncture. Catheter Cardiovasc Interv. 2013; 12. 82(7):1187–1192. PMID:
23592533.

11. Grier D, Hartnell G. Percutaneous femoral artery puncture: practice and anatomy. Br J Radiol. 1990; 8. 63(752):602–604. PMID:
2400874.

12. Grossman M. How to miss the profunda femoris. Radiology. 1974; 5. 111(2):482. PMID:
4818995.

13. Irani F, Kumar S, Colyer WR Jr. Common femoral artery access techniques: a review. J Cardiovasc Med (Hagerstown). 2009; 7. 10(7):517–522. PMID:
19412124.

14. Johnson LW, Esente P, Giambartolomei A, Grant WD, Loin M, Reger MJ, et al. Peripheral vascular complications of coronary angioplasty by the femoral and brachial techniques. Cathet Cardiovasc Diagn. 1994; 3. 31(3):165–172. PMID:
8025931.

15. Kim D, Orron DE, Skillman JJ, Kent KC, Porter DH, Schlam BW, et al. Role of superficial femoral artery puncture in the development of pseudoaneurysm and arteriovenous fistula complicating percutaneous transfemoral cardiac catheterization. Cathet Cardiovasc Diagn. 1992; 2. 25(2):91–97. PMID:
1544161.

16. Maecken T, Grau T. Ultrasound imaging in vascular access. Crit Care Med. 2007; 5. 35(5 Suppl):S178–S185. PMID:
17446777.

17. Schnyder G, Sawhney N, Whisenant B, Tsimikas S, Turi ZG. Common femoral artery anatomy is influenced by demographics and comorbidity: implications for cardiac and peripheral invasive studies. Catheter Cardiovasc Interv. 2001; 7. 53(3):289–295. PMID:
11458402.

18. Seto AH, Abu-Fadel MS, Sparling JM, Zacharias SJ, Daly TS, Harrison AT, et al. Real-time ultrasound guidance facilitates femoral arterial access and reduces vascular complications: FAUST (Femoral Arterial Access With Ultrasound Trial). JACC Cardiovasc Interv. 2010; 7. 3(7):751–758. PMID:
20650437.
19. Sherev DA, Shaw RE, Brent BN. Angiographic predictors of femoral access site complications: implication for planned percutaneous coronary intervention. Catheter Cardiovasc Interv. 2005; 6. 65(2):196–202. PMID:
15895402.

20. Stegemann E, Stegemann B, Marx N, Lauer T, Hoffmann R. Effect of preinterventional ultrasound examination on frequency of procedure-related vascular complications in percutaneous coronary interventions with transfemoral approach. Am J Cardiol. 2011; 11. 108(9):1203–1206. PMID:
21855839.

21. Tiroch KA, Arora N, Matheny ME, Liu C, Lee TC, Resnic FS. Risk predictors of retroperitoneal hemorrhage following percutaneous coronary intervention. Am J Cardiol. 2008; 12. 102(11):1473–1476. PMID:
19026298.

22. Wacker F, Wolf KJ, Fobbe F. Percutaneous vascular access guided by color duplex sonography. Eur Radiol. 1997; 11. 7(9):1501–1504. PMID:
9369522.

23. Yaganti V, Mejevoi N, Hasan O, Cohen M, Wasty N. Pitfalls associated with the use of current recommendations for fluoroscopy-guided common femoral artery access. Catheter Cardiovasc Interv. 2013; 3. 81(4):674–679. PMID:
23292908.

24. Yatskar L, Selzer F, Feit F, Cohen HA, Jacobs AK, Williams DO, et al. Access site hematoma requiring blood transfusion predicts mortality in patients undergoing percutaneous coronary intervention: data from the National Heart, Lung, and Blood Institute Dynamic Registry. Catheter Cardiovasc Interv. 2007; 6. 69(7):961–966. PMID:
17421023.

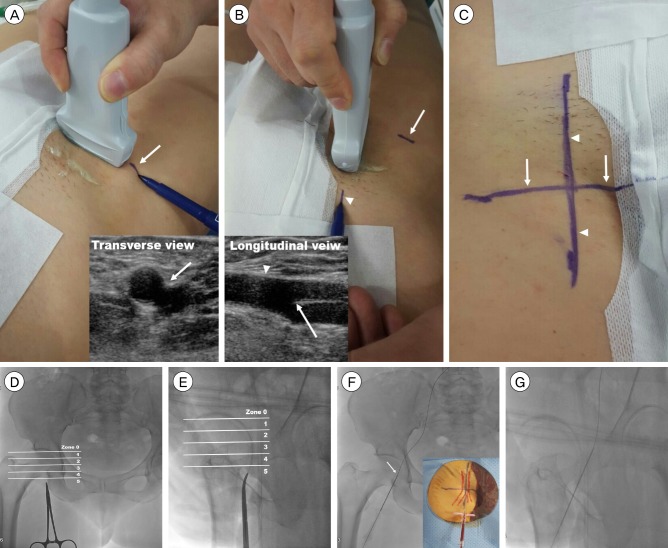
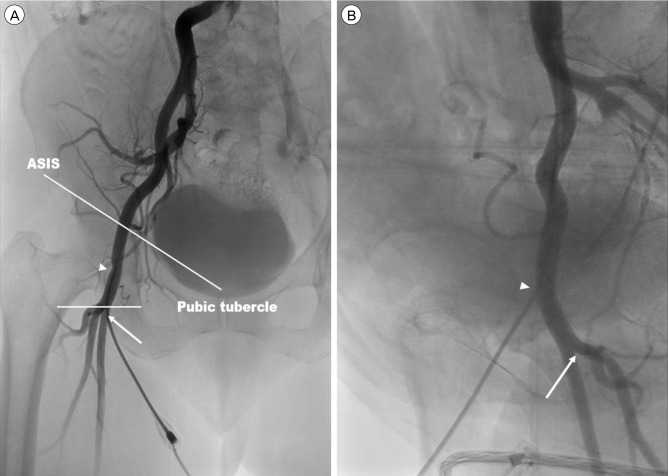
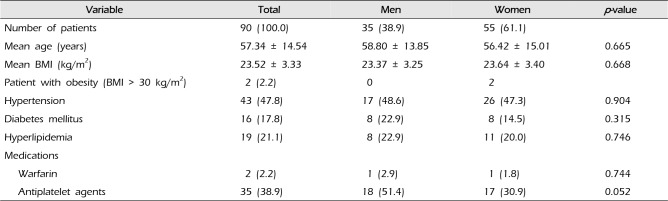
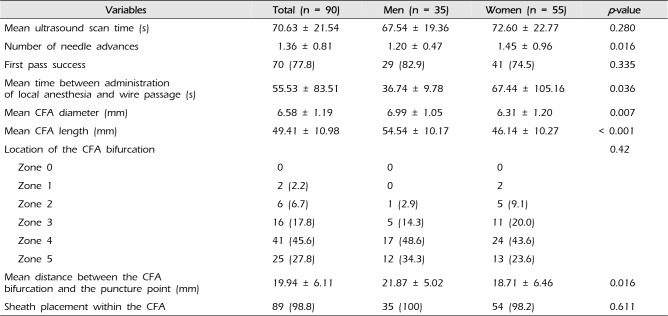




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download