This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
We evaluate the rates and outcomes of major procedure-related complications during coiling.
Materials and Methods
Between 2007 and 2015, 436 intracranial saccular aneurysms were treated. Complications are categorized as three types: intraprocedural aneurysm rupture (IAR), thromboembolism (TE), and post-procedural early rebleeding (PER). And we evaluated the risk factors of procedure related complications by multivariate analysis.
Results
Complications occurred in 61 aneurysms (14%). The overall incidence of complications in subarachnoid hemorrhage (SAH) was significantly higher than in unruptured intracranial aneurysm (UIA) (20% vs. 6%). The incidence of IAR and TE were higher in SAH than in UIA (IAR 12% vs. 4%, TE 7% vs. 3%, p < 0.05). Five PER occurred only in SAH. In 34 UIA which were treated with balloon-assisted coiling (BAC), all these patients had good recovery despite 3 patients had the IAR. The incidence of IAR and TE were not different between BAC and non-BAC groups (p > 0.05). All 7 patients who had IAR during BAC had good recovery. In multiple logistic regression analysis, female gender, SAH, and intraventricular hemorrhage were associated with procedure related complication (p < 0.05).
Conclusion
Endovascular coil embolization is a minimally invasive procedure, but incidence of its complication is not low, especially in SAH. BAC can be a good tool to avoid poor outcome from unexpected IAR during coiling. While IA tirofiban injection is a useful therapy in TE during coiling, sometimes we are aware of the risk of the early rebleeding in SAH patients.
Keywords: Aneurysm, Coil embolization, Intraprocedural aneurysm rupture, Thromboembolism
INTRODUCTION
Coil embolization is currently regarded as a safe and effective treatment for unruptured and ruptured intracranial aneurysms (IA) with low rates of procedure-related complications. However, bleeding or thromboembolism (TE) during procedure and post-procedural early rebleeding (PER), still remains the major concerns of coil embolization for endovascular neurosurgeon. The authors report the rates and outcomes of periprocedural complication during endovascular coiling on single center.
MATERIALS AND METHODS
Between July 2007 and November 2015, 436 intracranial saccular aneurysms were treated using simple coil, balloon-assisted coil (BAC), stent-assisted coil (SAC), and double microcatheter assisted coil (DAC) embolization at our institution. All complications were identified from the prospectively collected database. They are categorized into three types: intraprocedural aneurysm rupture (IAR), thromboembolism (TE), and PER. When the patient was stable, post-operative magnetic resonance imaging was routinely performed to detect the microemboli within 48 hours. Selection difficulty was defined when it had been already tried more than 3 times or when it takes longer than 20 minutes to select aneurysm. Clinical outcomes after 3 months were determined using Glasgow outcome scale (GOS) scores (1 = death, 2 = persistent vegetative state, 3 = severe disability requiring daily support, 4 = moderate disability but independent, and 5 = good recovery).
6) Poor outcome was defined as GOS score of < 4 at 3 months. Procedure related declined status was defined as any clinical deterioration evidence undergoing endovascular coiling by worsening of Glasgow coma scale (GCS) 2 or more during 1 month and the need for additional treatment such as craniotomy and placement of extraventricular drainage (EVD) within 48 hours.
Endovascular treatment
All procedures were performed under the general anesthesia and all ruptured aneurysms were treated within 48 hours of admission. For all patients with unruptured aneurysms in whom stent-assisted coil embolization was considered, dual antiplatelet therapy (aspirin 100 mg, clopidogrel 75 mg daily) was administered more than 1 week Prior to intervention. For ruptured aneurysms, loading doses of aspirin (300 mg) and clopidogrel (300 mg) were initiated via a nasogastric tube just before the stent deployment to prevent thromboembolic complications. Baseline activated coagulation time (ACT) was measured immediately after femoral puncture. Heparin was injected intravenously as a bolus (usually 3,000 unit), then additional 1,000 unit of heparin hourly basis to maintain ACT two to three times of baseline. Anticoagulation was initiated after femoral puncture in unruptured aneurysms and after intra-aneurysmal placement of the first coil in ruptured aneurysms. After procedure, the maintenance dose of dual antiplatelet drug (aspirin 100 mg, clopidogrel 75 mg daily) was used for 6 months and then maintained aspirin alone. BAC was employed when the aneurysm had a wide neck (neck diameter > 4 mm or dome-to neck ratio < 2). And when we use balloon in ruptured aneurysm, heparin was injected before balloon inflation. We usually use HyperForm balloon (ev3, Irvin, CA, USA), measuring 4 mm in maximal diameter and 7 mm in length.
Intraprocedural rupture
IAR was detected by direct fluoroscopic visualization of a device (coil, microwire, or microcatheter) outside the limits of the aneurysm or a new subarachnoid hemorrhage (SAH) on immediate post-operative computed tomography (CT) scanning or demonstration of extra-aneurysmal contrast leakage. As soon as IAR was detected, rapid coil packing to stop contrast leakage and heparin reversal with protamine sulfate were performed. Simultaneously we requested the modest decrease of blood pressure and the intravenous mannitol infusion for intracranial pressure (ICP) control to the anesthesiologist. And when the procedure was performed using a balloon, rapid inflation of balloon across the aneurysm neck and continued multiple coil packing without balloon deflation resulted in hemostasis. If placement of EVD was performed before the coil embolization, we opened the ventricular drain. According to the timing of IAR, we divided the 3 categories as follows: intraprocedural perforation of aneurysm during advancement of the microcatheter, during placement of first or second coils, during the 3rd or more coil placement. And we analyzed the prognosis with GOS, procedure related declined status.
Thromboembolism
Thrombus was diagnosed on native unsubtracted angiographic images and all patients were routinely checked with diffusion weighted imaging (DWI) on post-procedure. TE was defined as thrombus on angiogram during procedure or newly developed neurologic deficit with abnormal signal change on post-procedural DWI.
When thrombus formation or an embolic event were encountered during embolization, we continued coil packing until complete aneurysmal occlusion was achieved. And then intra-arterial (IA) tirofiban infusion was started when the thrombus burden was small or thrombus was located in the distal branches, or small parent artery such as anterior cerebral artery (ACA) or distal middle cerebral artery (MCA). If the thrombus burden was large or major large artery occlusion such as internal carotid artery (ICA) and basilar artery (BA), mechanical thrombectomy using stent retriever was tried.
We divided 2 groups of thromboembolic events; permanent TE and recanalized TE after thrombolysis or thrombectomy. Permanent TE was defined as the partially recanalized and remained thrombus despite thrombolysis, and recanalized TE was defined that flow was totally restored and no remnant thrombus was found after thrombolysis or observation.
In the case of a partially occluded parent artery, a clot was observed for 5 to 10 minutes. During the observation, ACT was checked and if it was less than twice the baseline value, additional 1,000 units of heparin was injected intravenously. A concentration of 0.05 mg/mL was made by a vial (12.5 mg / 50 mL) of tirofiban with normal saline and each 1 mL was injected during one minute. Average 0.2-1.2 mg was injected intra-arterially and a serial angiogram was performed until recanalization was obtained. In cases of remained thrombus on a parent artery or major artery (ICA, BA), mechanical thrombectomy was performed.
Statistical analysis
Quantitative variables are expressed as mean ± SD. We used Pearson's χ2 and Fisher's exact tests (when appropriate) analysis to test associations between categorical variables and t-tests or Mann-Whitney U tests for continuous variables. Univariate logistic regression analysis was also performed to detect significant associations of procedure-related complication. Only variables with values of p < 0.20 in the univariate analysis were included in the multivariable logistic regression model. Models were built by the use of backward stepwise logistic regression with variables entered into the model and removed at a 0.10 significance level. p values of < 0.05 were considered statistically significant. The odds ratio (OR) and 95% confidence interval (CI) were determined in the logistic regression analysis. Commercially available software (SPSS version 18, IBM Corp., Armonk, NY, USA) was used for all statistical analyses.
RESULTS
Demographic data
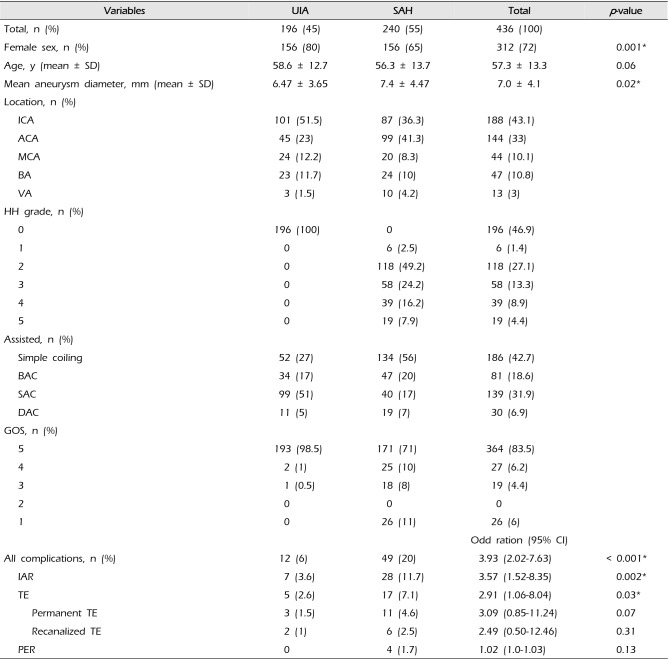
Table 1 summarizes the characteristics of the 436 patients. They consisted of 196 unruptured intracranial aneurysm (UIA) and 240 SAH (45% vs. 55%). Of 436 patients, female was 72%. Mean age was 57.3 years (range, 9–89 years). The mean clinical follow-up period was 30 months (range, 6–90 months). The common location of aneurysm were 188 (43%) in ICA and 144 (33%) in ACA. The treatment methods for 196 UIAs were as follows: SAC 99 (51%), simple coil 53 (27%), BAC 34 (17%), and DAC 11 (5%). And for 240 SAH coiling methods were as follows: simple coil 134 (56%), BAC 47 (20%), SAC 40 (17%), and DAC 19 (7%). All UIA patients achieved a good outcome except for one patient who had thromboembolic events. Overall mortality was 6% (0% in UIA and 11% in SAH).
Complications (IAR, TE, PER)
Complications occurred more common in SAH (20%) than UIA (6%) (OR 3.93; 95% CI 2.02-7.63,
p < 0.001). The IAR and TE occurred in 11.7 % and 7.1% of 240 SAH, respectively. All 5 PER were occurred in SAH.
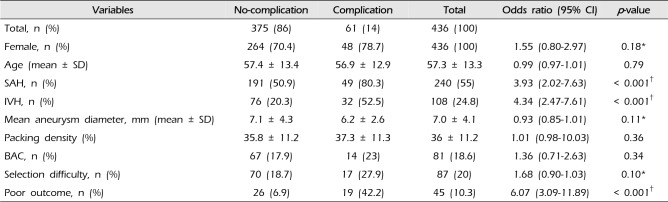
Table 2 shows the comparison results between no-complication and complication group occurring during endovascular coiling. In univariate analysis, SAH and IVH were statistically correlated with procedure-related complication (
p < 0.05). And procedure-related complication was strongly associated with poor outcome (GOS < 4) (unadjusted odds ratio [OR]: 6.07; 95% CI: 3.09–11.89;
p < 0.001). Although female gender, selection difficulty, and BAC were more common in complication group, there was no significant difference between two groups (
p > 0.05). Mean diameter of aneurysm in complication group was smaller than in no complication group (
p > 0.05).
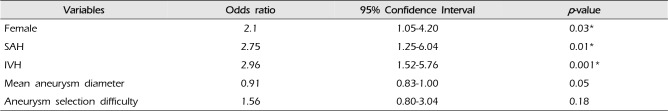
To identify the risk factor for complications during endovascular coiling, we analyzed the variables into the multiple logistic regression model (as shown in
Table 3). Multiple logistic regression analysis revealed that female gender, SAH and IVH were the independent risk factors for complications (adjusted OR: 2.75; 95% CI: 1.25–6.04;
p = 0.01, adjusted OR: 2.96; 95% CI: 1.52–5.76;
p = 0.001, respectively) and female was independently associated with complication (adjusted odds ratio [OR]: 2.1; 95% CI: 1.05–4.20;
p = 0.03).
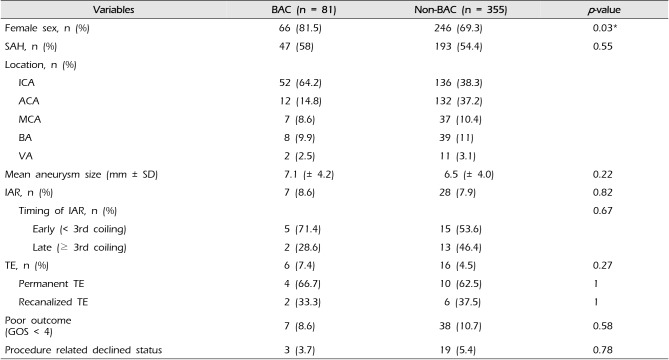
To evaluate the safety and efficacy of BAC, we categorized into the two groups: BAC (n = 81) group and non-BAC (n = 355) group.
Table 4 shows the baseline characteristics and results between two groups. There was no significant difference between two groups in distribution of SAH (58% vs. 54.4%; BAC vs. non-BAC,
p = 0.55). There was no significant difference in incidence of IAR and TE, poor outcome, and procedure related declined status between two groups (
p > 0.05).
While all 34 UIA which were treated with BAC had good recovery (GOD 5), 3 patients of 162 UIA without BAC had poor outcomes (GOS < 4). In 47 SAH with BAC, there were 11 complications (23.4%): 4 IAR, 6 TE (permanent 4, recanalized 2), and 1 PER, and 4 patients (8.5%) were dead and 3 patients (6.4%) had clinical deterioration. In 193 SAH without BAC, there were 38 complications (19.7%): 24 IAR, 11 TE (permanent 7, recanalized 4), and 3 PER and 22 patients (11.4%) were dead and 15 patients (7.8%) had clinical deterioration.
Intraprocedural aneurysm rupture (IAR)
IAR occurred in 35 cases (8%) of 436 aneurysms; it consisted of 7 (3.6%) in 196 UIA and 28 (11.7%) in 240 SAH (OR 3.57; 95% CI 1.52–8.35,
p < 0.001) (
Table 1). As for the location, these consisted of 15 ACA (43%), 10 ICA (29%), 6 BA (17%), 3 MCA (8.6%), and 1 VA (3%). According to the timing of IAR, there have been 4 cases of intraprocedural perforation of aneurysm during advancement of the microcatheter, 16 cases during placement of first or second coils, remaining 15 cases during the 3
rd or more coil placement. Although 20 patients had IAR on early stages (during microcatheter selection and 1
st & 2
nd coil insertion), 15 patients (72%) of these had good prognosis (GOS 4 & 5). Eight patients who were dead were already Hunt and Hess (HH) grade 4 or 5 on admission. Interestingly, all 7 IAR in UIA patients were achieved in a good recovery (GOS 5) at discharge and 3 of them were treated with BAC. If IAR occurred during BAC, hemostasis was obtained immediately using the inflation of balloon. And if IAR occurred during non-BAC, continuous coiling was performed with proper sized coils rapidly.
IAR occurred 7 patients (8.6%) in 81 BAC group and 28 patients (7.9%) in 355 non-BAC group (
p = 0.82) (
Table 4). In BAC, mostly a balloon was used from the beginning. One patient treated with a balloon for rescue after IAR and his GOS was 5 at discharge. As for the location, these consisted of 5 (71.4%) in p-com, 1 (14.3%) in a-com, and 1 (14.3%) in MCA. All patients in this group had good recovery (GOS 5) at discharge and during the follow up period. In non-BAC groups, stereotactic hematoma aspiration with EVD was performed in one patient with increasing ICH and SAH on post-operative CT. In this group, 8 patients were dead, but preoperative clinical status of these patients were already poor (HH 4 or 5). Procedure-related declined status occurred in none of BAC and 5 of non-BAC (0% vs. 17.9%,
p = 0.56).
Thromboembolism
Twenty-two (5%) patients had thromboembolic events during 436 endovascular coiling. There were permanent TE (n = 14, 60%) and recanalized TE (n = 8, 40%) after thrombolysis or thrombectomy. These consisted of 5 (2.5%) in 194 UIA and 17 (7%) in 240 SAH (OR 2.91; 95% CI 1.06-8.04,
p = 0.031) (
Table 1). These TE occurred in 7 (3.7%) of 186 simple coil, 6 (7.4%) of 81 BAC, 6 (4.3%) of 139 SAC, and 3 (10%) of 30 DCT. As for the location of thrombus, parent artery (73%) was most common site and symptomatic branch or perorating artery infarction with abnormal high signal intensity on post-procedural DWI (diffusion weighted image) occurred in 2 patients (9%). Thirteen patients (59%) with TE were treated with IA tirofiban infusion and 4 patients (18%) had observation and conservative treatment. Ten patients (45.5%) had the abnormal signal change on post-procedural DWI, and 6 patients (27%) of them had symptomatic infarction. Ipsilateral high signal intensity on post-procedural DWI and symptomatic infarctions were more common in permanent TE than in recanalized TE (
p > 0.05). And poor outcome in permanent TE was approximately 3 times more common in recanalized TE and procedure related declined status in permanent TE was 4 times more common in recanalized TE (
p > 0.05).
Even though TE occurred more common in BAC group than non-BAC (7.4% vs 4.5%), the difference was not statistically significant (
p = 0.27,
Table 4). In BAC group, all patients who had TE were in SAH, but there was no significant difference in distribution of SAH between two groups (
Table 4).
Post-procedure early bleeding
Four patients (0.9%) had PER after endovascular treatment. All 4 patients were SAH and female, and these locations consisted of two distal ACA aneurysms, 1 pcom aneurysm and 1 MCA aneurysm. (
Table 1). There were no selection difficulty with microcatheter and no angiospasm. Mean packing density was 39% and post-procedural according to the Raymond obliteration degree, were were I in 1, II in 2, and III in 1. Procedure related declined status was in all 4 patients (80%). Mean heparin dose was 4,500 units. All 4 cases were related to medication (IA tirofiban, continuous IV heparinization) or insufficient coil packing. IA tirofiban or IV continuous heparin injection to prevent the TE for herniation of coil loop made a PER. The rebleeding occurred within 24 hours in all cases and 2 patients underwent the decompressive craniectomy and aneurysm neck clipping after coil removal. One patient was dead on postoperative day 7 and 3 patients (75%) had procedure related declined status.
DISCUSSION
Recently coil embolization is regarded as a safe procedure and is the primary treatment option for IA. In a comparative study between coiling and clipping on ruptured aneurysms, coil embolization is recommended if the aneurysm is considered suitable for both surgical clipping and endovascular treatment.
12) The advantages of coil embolization over surgical clipping vary widely according to aneurysm location, but endovascular treatment has benefits for all sites. Coil embolization results in a lower incidence of hospital mortality and shorter hospital stays than surgical clipping. Although coil yields better clinical outcomes especially in a good preoperative grade patient, coiling leads to a greater risk of some complications. The incidence of rebleeding is higher after coiling than clipping, and clipping has a better complete occlusion rate.
8) Some studies reported that complications associated with coil embolization ranged from 8.6% to 18.6% with median about 10.6% and a higher complication rate in coil embolization than in surgical clipping (16% vs. 6.8%).
5) Brilstra et al. reported that total complication rate with coil embolization was 12% and the rate of permanent complication was 3.7%.
2) Coil embolization has many complications as follows; TE, perforation of aneurysm (IAR), parent artery obstruction, collapsed coils, coil malposition, coil migration and PER.
Intraprocedural aneurysm rupture
In our study, IAR occurred in 8% of 436 aneurysms; the rate of IAR was higher in ruptured than unruptured aneurysms (11.7% vs. 3.6%). According to the literature, the rate of IAR varies, ranging from 2.3% to 4.7% and its incidence was more frequent in ruptured aneurysms and in pushable coils.
1) Cloft and Kallmes reported that the risk of perforation during embolization was higher in a patient with a previously ruptured aneurysm (4.1% vs. 0.7%) and perforation of aneurysm during coil embolization tended to result in increasing the morbidity and mortality.
4) Previously ruptured aneurysms are very unstable on hemodynamic and the wall is fragile due to inflammatory reaction, so that subtle motion or pressure to the aneurysm wall could make an IAR. In general, IAR provides a poor outcome, but the outcome of 7 patients who had IAR during BAC showed good recovery in our study. Its reason was that BAC is seems to be a protective role in IAR. Balloon inflation in IAR prevented the increasing SAH and ICH with flow arrest, so that BAC was clinically beneficial during the immediate post-procedural period. Some argument that BAC encourage the IAR during coiling is still controversial. However, BAC seems to be a useful tools in IAR, although the incidence of IAR poor outcome and procedure related declined status were not significantly different between BAC and non-BAC group in our study.
Thromboembolism
TE is one of major complications of coil embolization with an incidence of 8 to 28%.
3)9)11) In our series, 5% occurred in TE during coiling. We tried to do coiling without stenting in SAH for preventing TE, so 76% of ruptured aneurysms were performed by simple coiling (56%) or BAC (20%) in our study. Although it remains a matter of debate, recent study showed that BAC is not associated with increasing the risk of procedure-related morbidity or mortality from other complications like TE.
10) Our study also showed no statistical difference in incidence of TE between BAC and non-BAC (7.4% vs. 4.5,
p = 0.28). The reason why incidence of TE had no difference between BAC and non-BAC despite flow arrest or stasis occurred during balloon inflation might be the safe protocol of antiplatelet and anticoagulant. Routinely we did IV heparinization before inflation of the balloon regardless of SAH. Early heparinization and administration of dual antiplatelet therapy for at least 5 days before embolization of UIA prevented the TE sufficiently. When TE occurred, IA tirofiban injection was a common method for rescue.
7) Despite tirofiban injection and mechanical thrombectomy being performed, the rate of total recanalization was obtained as 40% in our study. On the other hands, all 6 patients who had TE during BAC were in SAH. If BAC has a risk of TE in SAH which has hypercoagulable state in acute phase, we need more time to study about it.
In our study, BAC did not increase the IAR or TE during procedure (
Table 4). Besides, it could prevent poor outcome after IAR. BAC is very safe and effective in Acom and Pcom aneurysm coiling.
Post-procedure early bleeding
In our study, PER occurred in 0.9% of 436 IAs within 24 hours. All these patients were in SAH, and mostly had poor outcome course (75%). The most common reason of PER were antiplatelet or anticoagulant therapy (heparin and tirofiban). Despite total or subtotal coil packing being performed finally, the use of IA tirofiban during procedure resulted in ICH and brain edema. One patient had massive early rebleeding after coiling because of continuous injection of IV heparin to prevent TE after coil loop herniation which developed in the end stage of coiling. If TE occurred when coiling had not finished, continuous coil packing should be quickly performed to reach excessive packing density before tirofiban injection. Over coil packing reduced the chance of PER after using the antiplatelet and anticoagulant during the TE event.
7) As the treatment of mild coil loop herniation to prevent the post-procedural TE, aspirin alone or dual antiplatelet therapy are the better choice than continuous IV heparin injection on post-procedural period.
CONCLUSION
Although coil embolization is now a leading and feasible option for aneurysm treatment, sometimes any event can provide a poor outcome according to inappropriate decision making. Incidence of its complication in SAH is not low. BAC is the useful therapy on endovascular coiling without increasing the incidence of IAR or TE, it is a good rescue therapy on IAR. The use of thrombolytic agents seems to be a double-edged sword. While IA tirofiban is useful therapy in TE during coiling, sometimes it could produce early rebleeding on inappropriate use. We should always consider the use of thrombolytic agents carefully on facing thromboembolic events.
ACKNOWLEDGEMENTS
This research was supported by the Soonchunhyang University Research Fund.
References
1. Barker FG 2nd, Amin-Hanjani S, Butler WE, Hoh BL, Rabinov JD, Pryor JC, et al. Age-dependent differences in short-term outcome after surgical or endovascular treatment of unruptured intracranial aneurysms in the United States, 1996-2000. Neurosurgery. 2004; 1. 54(1):18–28. PMID:
14683537.

2. Brilstra EH, Rinkel GJ, van der Graaf Y, van Rooij WJ, Algra A. Treatment of intracranial aneurysms by embolization with coils: a systematic review. Stroke. 1999; 2. 30(2):470–476. PMID:
9933290.
3. Brinjikji W, McDonald JS, Kallmes DF, Cloft HJ. Rescue treatment of thromboembolic complications during endovascular treatment of cerebral aneurysms. Stroke. 2013; 5. 44(5):1343–1347. PMID:
23598522.

4. Cloft HJ, Kallmes DF. Cerebral aneurysm perforations complicating therapy with Guglielmi detachable coils: a meta-analysis. AJNR Am J Neuroradiol. 2002; Nov-Dec. 23(10):1706–1709. PMID:
12427628.
5. Health Quality Ontario. Coil embolization for intracranial aneurysms: an evidence-based analysis. Ont Health Technol Assess Ser. 2006; 6(1):1–114.
6. Jennett B, Snoek J, Bond MR, Brooks N. Disability after severe head injury: observations on the use of the Glasgow Outcome Scale. J Neurol Neurosurg Psychiatry. 1981; 4. 44(4):285–293. PMID:
6453957.

7. Kang HS, Kwon BJ, Roh HG, Yoon SW, Chang HW, Kim JE, et al. Intra-arterial tirofiban infusion for thromboembolism during endovascular treatment of intracranial aneurysms. Neurosurgery. 2008; 8. 63(2):230–237. discussion 237-8. PMID:
18797352.

8. Li H, Pan R, Wang H, Rong X, Yin Z, Milgrom DP, et al. Clipping versus coiling for ruptured intracranial aneurysms: a systematic review and meta-analysis. Stroke. 2013; 1. 44(1):29–37. PMID:
23238862.
9. Park HK, Horowitz M, Jungreis C, Genevro J, Koebbe C, Levy E, et al. Periprocedural morbidity and mortality associated with endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol. 2005; 3. 26(3):506–514. PMID:
15760857.
10. Pierot L, Spelle L, Leclerc X, Cognard C, Bonafe A, Moret J. Endovascular treatment of unruptured intracranial aneurysms: comparison of safety of remodeling technique and standard treatment with coils. Radiology. 2009; 6. 251(3):846–855. PMID:
19318586.

11. Ross IB, Dhillon GS. Complications of endovascular treatment of cerebral aneurysms. Surg Neurol. 2005; 7. 64(1):12–18. PMID:
15993171.

12. van der Schaaf I, Algra A, Wermer M, Molyneux A, Clarke M, van Gijn J, et al. Endovascular coiling versus neurosurgical clipping for patients with aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2005; (4):CD003085. PMID:
16235314.

Table 1
Demographics of 436 treated intracranial saccular aneurysms
|
Variables |
UIA |
SAH |
Total |
p-value |
|
Total, n (%) |
196 (45) |
240 (55) |
436 (100) |
|
|
Female sex, n (%) |
156 (80) |
156 (65) |
312 (72) |
0.001*
|
|
Age, y (mean ± SD) |
58.6 ± 12.7 |
56.3 ± 13.7 |
57.3 ± 13.3 |
0.06 |
|
Mean aneurysm diameter, mm (mean ± SD) |
6.47 ± 3.65 |
7.4 ± 4.47 |
7.0 ± 4.1 |
0.02*
|
|
Location, n (%) |
|
|
|
|
|
ICA |
101 (51.5) |
87 (36.3) |
188 (43.1) |
|
ACA |
45 (23) |
99 (41.3) |
144 (33) |
|
MCA |
24 (12.2) |
20 (8.3) |
44 (10.1) |
|
BA |
23 (11.7) |
24 (10) |
47 (10.8) |
|
VA |
3 (1.5) |
10 (4.2) |
13 (3) |
|
HH grade, n (%) |
|
|
|
|
|
0 |
196 (100) |
0 |
196 (46.9) |
|
1 |
0 |
6 (2.5) |
6 (1.4) |
|
2 |
0 |
118 (49.2) |
118 (27.1) |
|
3 |
0 |
58 (24.2) |
58 (13.3) |
|
4 |
0 |
39 (16.2) |
39 (8.9) |
|
5 |
0 |
19 (7.9) |
19 (4.4) |
|
Assisted, n (%) |
|
|
|
|
|
Simple coiling |
52 (27) |
134 (56) |
186 (42.7) |
|
BAC |
34 (17) |
47 (20) |
81 (18.6) |
|
SAC |
99 (51) |
40 (17) |
139 (31.9) |
|
DAC |
11 (5) |
19 (7) |
30 (6.9) |
|
GOS, n (%) |
|
|
|
|
|
5 |
193 (98.5) |
171 (71) |
364 (83.5) |
|
4 |
2 (1) |
25 (10) |
27 (6.2) |
|
3 |
1 (0.5) |
18 (8) |
19 (4.4) |
|
2 |
0 |
0 |
0 |
|
1 |
0 |
26 (11) |
26 (6) |
|
|
|
Odd ration (95% CI) |
|
All complications, n (%) |
12 (6) |
49 (20) |
3.93 (2.02–7.63) |
< 0.001*
|
|
IAR |
7 (3.6) |
28 (11.7) |
3.57 (1.52–8.35) |
0.002*
|
|
TE |
5 (2.6) |
17 (7.1) |
2.91 (1.06–8.04) |
0.03*
|
|
Permanent TE |
3 (1.5) |
11 (4.6) |
3.09 (0.85–11.24) |
0.07 |
|
Recanalized TE |
2 (1) |
6 (2.5) |
2.49 (0.50–12.46) |
0.31 |
|
PER |
0 |
4 (1.7) |
1.02 (1.0–1.03) |
0.13 |
Table 2
Comparison results between no-complication and complication group
|
Variables |
No-complication |
Complication |
Total |
Odds ratio (95% CI) |
p-value |
|
Total, n (%) |
375 (86) |
61 (14) |
436 (100) |
|
|
|
Female, n (%) |
264 (70.4) |
48 (78.7) |
436 (100) |
1.55 (0.80–2.97) |
0.18*
|
|
Age (mean ± SD) |
57.4 ± 13.4 |
56.9 ± 12.9 |
57.3 ± 13.3 |
0.99 (0.97–1.01) |
0.79 |
|
SAH, n (%) |
191 (50.9) |
49 (80.3) |
240 (55) |
3.93 (2.02–7.63) |
< 0.001†
|
|
IVH, n (%) |
76 (20.3) |
32 (52.5) |
108 (24.8) |
4.34 (2.47–7.61) |
< 0.001†
|
|
Mean aneurysm diameter, mm (mean ± SD) |
7.1 ± 4.3 |
6.2 ± 2.6 |
7.0 ± 4.1 |
0.93 (0.85–1.01) |
0.11*
|
|
Packing density (%) |
35.8 ± 11.2 |
37.3 ± 11.3 |
36 ± 11.2 |
1.01 (0.98–10.03) |
0.36 |
|
BAC, n (%) |
67 (17.9) |
14 (23) |
81 (18.6) |
1.36 (0.71–2.63) |
0.34 |
|
Selection difficulty, n (%) |
70 (18.7) |
17 (27.9) |
87 (20) |
1.68 (0.90–1.03) |
0.10*
|
|
Poor outcome, n (%) |
26 (6.9) |
19 (42.2) |
45 (10.3) |
6.07 (3.09–11.89) |
< 0.001†
|
Table 3
Risk factors for procedure-related complications by multiple logistic regression analysis in 436 patients who treated endovascular coiling for intracranial saccular aneurysm
|
Variables |
Odds ratio |
95% Confidence Interval |
p-value |
|
Female |
2.1 |
1.05–4.20 |
0.03*
|
|
SAH |
2.75 |
1.25–6.04 |
0.01*
|
|
IVH |
2.96 |
1.52–5.76 |
0.001*
|
|
Mean aneurysm diameter |
0.91 |
0.83–1.00 |
0.05 |
|
Aneurysm selection difficulty |
1.56 |
0.80–3.04 |
0.18 |
Table 4
Comparison results between BAC and non-BAC in 436 intracranial aneurysms
|
Variables |
BAC (n = 81) |
Non-BAC (n = 355) |
p-value |
|
Female sex, n (%) |
66 (81.5) |
246 (69.3) |
0.03*
|
|
SAH, n (%) |
47 (58) |
193 (54.4) |
0.55 |
|
Location, n (%) |
|
|
|
|
ICA |
52 (64.2) |
136 (38.3) |
|
ACA |
12 (14.8) |
132 (37.2) |
|
MCA |
7 (8.6) |
37 (10.4) |
|
BA |
8 (9.9) |
39 (11) |
|
VA |
2 (2.5) |
11 (3.1) |
|
Mean aneurysm size (mm ± SD) |
7.1 (± 4.2) |
6.5 (± 4.0) |
0.22 |
|
IAR, n (%) |
7 (8.6) |
28 (7.9) |
0.82 |
|
Timing of IAR, n (%) |
|
|
0.67 |
|
Early (< 3rd coiling) |
5 (71.4) |
15 (53.6) |
|
Late (≥ 3rd coiling) |
2 (28.6) |
13 (46.4) |
|
TE, n (%) |
6 (7.4) |
16 (4.5) |
0.27 |
|
Permanent TE |
4 (66.7) |
10 (62.5) |
1 |
|
Recanalized TE |
2 (33.3) |
6 (37.5) |
1 |
|
Poor outcome (GOS < 4) |
7 (8.6) |
38 (10.7) |
0.58 |
|
Procedure related declined status |
3 (3.7) |
19 (5.4) |
0.78 |








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download