Abstract
Objective
The cause of severe clinical vasospasm after aneurysmal subarachnoid hemorrhage remains unknown, despite extensive research over the past 30 years. However, the intra-arterial administration of vasodilating agents and balloon angioplasty have been successfully used in severe refractory cerebral vasospasm.
Materials and Methods
We retrospectively analyzed the data of 233 patients admitted to our institute with aneurysmal subarachnoid hemorrhage (SAH) over the past 3 years.
Results
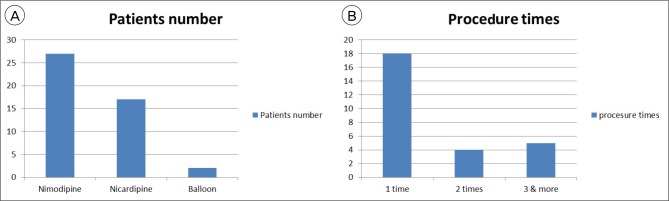
Of these, 27 (10.6%) developed severe symptomatic vasospasm, requiring endovascular therapy. Vasospasm occurred at an average of 5.3 days after SAH. A total of 46 endovascular procedures were performed in 27 patients. Endovascular therapy was performed once in 18 (66.7%) patients, 2 times in 4 (14.8%) patients, 3 or more times in 5 (18.5%) patients. Intra-arterial vasodilating agents were used in 44 procedures (27 with nimodipine infusion, 17 with nicardipine infusion). Balloon angioplasty was performed in only 2 (7.4%) patients. The Average nimodipine infusion volume was 2.47 mg, and nicardipine was 3.78 mg. Most patients recovered after the initial emergency room visit. Two patients (7.4%) worsened, but there were no deaths.
Cerebral vasospasm remains a leading cause of morbidity and mortality following aneurysmal subarachnoid hemorrhage (SAH).21) Cerebral vasospasm is a prolonged but reversible narrowing of cerebral arteries beginning days after SAH. Progression to cerebral ischemia is tied mostly to vasospasm severity, and its pathogenesis lies in artery encasement by blood clots, although the complex interactions between hematoma and surrounding structures are not fully understood. Minimizing ischemia by avoiding inadequate blood volume and pressure, administering the calcium antagonist nimodipine, and intervention with balloon angioplasty, when necessary, constitutes the current best management.13) The objective of endovascular treatment of cerebral arterial vasospasm is to restore adequate blood flow and to prevent cerebral ischemia and infarction.41) Two different techniques are applied: mechanical or pharmacological vessel dilatation. Mechanical vasodilatation is achieved by means of transluminal balloon angioplasty and is directed towards a circumscribed proximal vessel segment. Pharmacological endovascular dilatation is based on continuous intraarterial infusion of drug via a microcatheter positioned within the proximal intracranial vessel territory affected by vasospasm.41) However, vasospasm remains an important determinant of outcome after aneurysm rupture. The aim of this study was to analyze the incidence and results of endovascular treatment of vasospasm.
A total of 233 patients with aneurysmal SAH were enrolled in this study between January 2012 and December 2014. Patient information and endovascular treatment methods are summarized in Table 1. All patients underwent aneurysm clipping or coil embolization to prevent rebleeding. Vasospasm was confirmed by performing computed tomography (CT) angiography or digital subtraction angiography in symptomatic patients.
In our series of 233 SAH patients, 27 (11.6%) developed severe clinical vasospasm requiring endovascular therapy. Vasospasm occurred at an average of 5.3 days after SAH. A total of 46 endovascular procedures were performed in 27 patients. Intra-arterial injections of nimodipine (average dose, 2.47 mg) or nicardipine (average dose 3.78 mg) was performed 44 times in 27 patients and balloon angioplasty was performed in 2 patients. Endovascular therapy was performed once in 18 (66.7%) patients, 2 times in 4 (14.8%) patients, and 3 or more times in 5 (18.5%) patients (Fig. 1).
The most frequent site of aneurysm was the anterior communicating artery, followed by the posterior communicating artery (26.2%) and middle cerebral artery (18.9%) (Table 2, Fig. 2). The most frequent site of aneurysms in symptomatic vasospasm patients was also anterior communicating artery (37.1%), followed by posterior communicating artery (25.9%) and middle cerebral artery (18.5%) (Table 3, Fig. 3).
The clinical results are shown in Table 4. Improvement was observed in 14 (51.9%) patients. Craniectomy was performed in 4 (14.8%). The mortality rate was 11.1%. The causes of death were pneumonia in 2 patients (7.4%) and acute renal failure in 1 patient (3.7%). After the patients were followed-up for 3–6 months, favorable outcomes were observed (average modified Rankin scale score: 3.3). No procedure-related complications were observed.
Angiographic vasospasm is common after rupture of an aneurysm, with an overall incidence of 50–90%.9) Vasospasm is rare in the first 2 days after SAH, but increases after the fourth day.27) In 530 cases,28) the highest incidence was between days 10 and 17, peaking on day 13 at 60%. In another series,16) vasospasm occurred in a third of patients in the first week and in two-thirds in the second week. Accordingly, it is important to remain vigilant for this complication for at least 2 weeks after SAH. The aim of vasospasm management is to optimize cerebral blood flow in order to prevent delayed cerebral ischemia (DCI). Medical treatment is usually the initial step when vasospasm is suspected. However, vasospasm is often refractory to these treatments. Moreover, many patients do not tolerate the combination of induced hypertension, hypervolemia, and hemodilution, which is referred to as ‘triple-H’ therapy, usually due to cardiac and pulmonary complications including myocardial ischemia, congestive heart failure and pulmonary edema. For these patients, early endovascular treatment appears to be the best alternative.1)12)29)31)39)44)
Papaverine was first described as a treatment option for cerebral vasospasm in 1992.19)20) Its mechanism is believed to result from inhibition of cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate phosphodiesterase in the smooth muscle, as well as from blockage of calcium ion channels in the cell membrane, resulting in vasodilation by inhibition of smooth muscle contraction. Firlik et al.15) analyzed the use of papaverine in 15 patients with symptomatic vasospasm after SAH. The vessel caliber improved immediately following treatment on 18/23 occasions. Vasospasm associated clinical symptoms improvement was major on 6 cases, and either minor or nonexistent on 17. The authors concluded that although the papaverine was reduced in the reversal of arterial narrowing in the majority of cases (78%), this angiographic improvement was associated with CBF augmentation in only 46% of the cases and major clinical improvement in only 26%.15) Papaverine treatment is effective within the entire vessel territory, often treated and recirculated exerts an effect within other vascular territories as well. Its action is predominantly directed towards the active component of arterial vasospasm.
Nimodipine was first tested as a treatment for DCI due to its preferential vasodilation of the cerebral circulation and experimental work showing reduced impairment of post ischemic reperfusion.23) Nimodipine is an L-type dihydropyridine calcium channel antagonist and remains the only intervention shown to consistently reduce the incidence of DCI and improve outcomes after SAH. Calcium plays a critical role in cellular communication and regulation and is important for the maintenance of normal cerebral vascular tone.36) Nimodipine has a neuroprotective effect in SAH by blocking calcium influx after tissue ischemia at a neuronal level.32)38) However, as nimodipine does not exert a beneficial effect on other diseases that lead to cerebral ischemia such as ischemic stroke18) or traumatic brain injury,28) it seems likely that it acts on a mechanism specific to SAH. Potential complications include hypotension, bradycardia, rash and diarrhea. Rare cases of refractory hypotension leading to death have been reported with the intravenous use of nimodipine.4) An experimental SAH model suggested that intra-arterial nimodipine might be more effective than intra-arterial papaverine in promoting reversal of vasospasm.2)14) Biondi et al.6) studied the efficacy of intra-arterial nimodipine in preventing infarcts in 25 consecutive patients with symptomatic vasospasm.
Nicardipine is a dihydropyridine calcium antagonist, similar to nimodipine, blocks the L-type voltage-gated calcium channels, preventing the influx of extracellular calcium, which would eventfully lead to muscle contraction. Badjatia et al.3) reported IA nicardipine in the treatment of SAH-induced vasospasm. Forty-four vessels were treated in 18 patients. All vessels treated demonstrated angiographic improvement in the degree of vasospasm. TCD demonstrated significant decreases in peak systolic velocity after treatment. Neurological improvement was seen in about 42% of the patients after treatment. No significant changes in blood pressure or signs of pulmonary edema or renal dysfunction.
Balloon angioplasty is usually limited to the proximal vessels (> 2–3 mm), predominantly the supraclinoid internal carotid artery, the M1 and proximal M2 segments of the middle cerebral artery, the A1 segment of the anterior cerebral artery, the P1 segment of the posterior cerebral artery, and the basilar artery. Distal vessel balloon angioplasty is typically not feasible. Vessel tortuosity, preventing endovascular navigation, is another potential limitation.8) In a review by Hoh and Ogilvy,17) balloon angioplasty had an approximately 5% risk of a major complication, including an approximately 1% risk of vessel perforation/rupture, which is usually fatal.5)10)11)17)31)32)33)39)47)48) Even though angioplasty is directed to and effective on a proximal vessel segments only, the rational is to improve blood flow, oxygenation and metabolism within the distal arterial territory as well.15) Balloon angioplasty is superior to chemical angioplasty for the permanent treatment of vasospasm.22) The durable effects of angioplasty after stretching of the vessel wall are well documented,7)25) whereas the vasodilatory action of papaverine is short lasting.26)34)
Numerous pharmacologic agents have been tested in experimental models of SAH and vasospasm, many of which were intended to explore a particular pathogenic theory for vasospasm by targeting a specific pathologic process. For example, ET-1 overproduction by cerebral endothelial cells damaged by SAH is a leading theory in the pathogenesis of cerebral vasospasm. The ETA/B receptor antagonist TAK-044 was found to reduce DCI with an acceptable safety profile in a phase II randomized controlled trial, but with no apparent effect on outcome.37)42) The ETA receptor antagonist clazosentan, which showed promising results in a multicenter phase IIa study,46) has now been studied in a randomized, double-blind, placebo-controlled, phase II dose-finding trial (Clazosentan to Overcome Neurological iSChemia and Infarct OccUrring after Subarachnoid hemorrhage [CONSCIOUS-1]).30) Statins increase nitric oxide biosynthesis and bioavailability, and have great potential for vasospasm prophylaxis in the setting of SAH.35) A meta-analysis of 6 randomized controlled statin trials found no overall significant reduction in poor neurological outcome, although delayed ischemic deficits were less common in the statin-treated patients.45) The international, randomized, double-blind Simvastatin in Aneurysmal Subarachnoid Hemorrhage (STASH) trial recruited 803 patients until February 2013. This study failed to detect any short- or long-term benefit in the use of simvastatin 40 mg per day, started within 96 hours of stroke, followed by up to 3 weeks of administration.24) Magnesium sulfate (MgSO4) has neuroprotective and vasodilatory properties and has therefore been tested for the prevention of vasospasm and ischemia in patients with SAH. One early study found no benefit from intravenous MgSO4 infusions,47) another showed a variety of beneficial trends toward benefit,43) and a third suggested efficacy equivalent to nimodipine in preventing ischemic damage.40)
Endovascular intervention may have an important role in selected cases to reduce vasospasm-related morbidity and mortality after aneurysmal SAH. In our experience, endovascular treatment is safe, with a low complication rate, but further studies are required to determine appropriate patient selection and treatment efficacy.
References
1. Awad IA, Carter LP, Spetzler RF, Medina M, Williams FC Jr. Clinical vasospasm after subarachnoid hemorrhage: response to hypervolemic hemodilution and arterial hypertension. Stroke. 1987; Mar-Apr. 18(2):365–372. PMID: 3564092.

2. Barth M, Capelle HH, Weidauer S, Weiss C, Münch E, Thomé C, et al. Effect of nicardipine prolonged-release implants on cerebral vasospasm and clinical outcome after severe aneurysmal subarachnoid hemorrhage: a prospective, randomized, double-blind phase IIa study. Stroke. 2007; 2. 38(2):330–336. PMID: 17185636.
3. Badjatia N, Topcuoglu MA, Pryor JC, Rabinov JD, Ogilvy CS, Carter BS, et al. Preliminary experience with intra-arterial nicardipine as a treatment for cerebral vasospasm. AJNR Am J Neuroradiol. 2004; 5. 25(5):819–826. PMID: 15140728.
4. Bayer HealthCare: Important drug warning. 2006. 2.
5. Bejjani GK, Bank WO, Olan WJ, Sekhar LN. The efficacy and safety of angioplasty for cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 1998; 5. 42(5):979–986. discussion 986-7. PMID: 9588541.

6. Biondi A, Ricciardi GK, Puybasset L, Abdennour L, Longo M, Chiras J, et al. Intra-arterial nimodipine for the treatment of symptomatic cerebral vasospasm after aneurysmal subarachnoid hemorrhage: preliminary results. AJNR Am J Neuroradiol. 2004; Jun-Jul. 25(6):1067–1076. PMID: 15205150.
7. Chan PD, Findlay JM, Vollrath B, Cook DA, Grace M, Chen MH, et al. Pharmacological and morphological effects of in vitro transluminal balloon angioplasty on normal and vasospastic canine basilar arteries. J Neurosurg. 1995; 9. 83(3):522–530. PMID: 7666232.

8. Dabus G, Nogueira RG. Current options for the management of aneurysmal subarachnoid hemorrhage-induced cerebral vasospasm: A comprehensive review of the literature. Interv Neurol. 2013; 10. 2(1):30–51. PMID: 25187783.

9. Dorsch NW, King MT. A review of cerebral vasospasm in aneurysmal subarachnoid haemorrhage: Part I: Incidence and effects. J Clin Neurosci. 1994; 1. 1(1):19–26. PMID: 18638721.

10. Elliott JP, Newell DW, Lam DJ, Eskridge JM, Douville CM, Le Roux PD, et al. Comparison of balloon angioplasty and papaverine infusion for the treatment of vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 1998; 2. 88(2):277–284. PMID: 9452236.

11. Eskridge JM, McAuliffe W, Song JK, Deliganis AV, Newell DW, Lewis DH, et al. Balloon angioplasty for the treatment of vasospasm: results of first 50 cases. Neurosurgery. 1998; 3. 42(3):510–516. discussion 516-7. PMID: 9526985.
12. Feigin VL, Rinkel GJ, Algra A, Vermeulen M, van Gijn J. Calcium antagonists in patients with aneurysmal subarachnoid hemorrhage: a systematic review. Neurology. 1998; 4. 50(4):876–883. PMID: 9566366.

13. Findlay JM, Nisar J, Darsaut T. Cerebral vasospasm: A review. Can J Neurol Sci. 2016; 1. 43(1):15–32. PMID: 26332908.

14. Firat MM, Gelebek V, Orer HS, Belen D, Firat AK, Balkanci F. Selective intraarterial nimodipine treatment in an experimental subarachnoid hemorrhage model. AJNR Am J Neuroradiol. 2005; Jun-Jul. 26(6):1357–1362. PMID: 15956497.
15. Firlik KS, Kaufmann AM, Firlik AD, Jungreis CA, Yonas H. Intra-arterial papaverine for the treatment of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Surg Neurol. 1999; 1. 51(1):66–74. PMID: 9952126.

16. Griffith HB, Cummains BH, Thomson JL. Cerebral arterial spasm and hydrocephalus in leaking arterial aneurysms. Neuroradiology. 1972; 12. 4(4):212–214. PMID: 4677616.

17. Hoh BL, Ogilvy CS. Endovascular treatment of cerebral vasospasm: transluminal balloon angioplasty, intraarterial papaverine, and intra-arterial nicardipine. Neurosurg Clin N Am. 2005; 7. 16(3):501–516. viPMID: 15990041.

18. Horn J, Limburg M. Calcium antagonists for ischemic stroke: a systematic review. Stroke. 2001; 2. 32(2):570–576. PMID: 11157199.
19. Kaku Y, Yonekawa Y, Tsukahara T, Kazekawa K. Superselective intra-arterial infusion of papaverine for the treatment of cerebral vasospasm after subarachnoid hemorrhage. J Neurosurg. 1992; 12. 77(6):842–847. PMID: 1432124.

20. Kassell NF, Helm G, Simmons N, Phillips CD, Cail WS. Treatment of cerebral vasospasm with intra-arterial papaverine. J Neurosurg. 1992; 12. 77(6):848–852. PMID: 1432125.

21. Kassell NF, Torner JC, Haley EC Jr, Jane JA, Adams HP, Kongable GL. The international cooperative study on the timing of aneurysm surgery. Part 1: overall management results. J Neurosurg. 1990; 7. 73(1):18–36. PMID: 2191090.
22. Katoh H, Shima K, Shimizu A, Takiguchi H, Miyazawa T, Umezawa H, et al. Clinical evaluation of the effect of percutaneous transluminal angioplasty and intra-arterial papaverine infusion for the treatment of vasospasm following aneurysmal subarchnoid hemorrhage. Neurol Res. 1999; 3. 21(2):195–203. PMID: 10100208.
23. Kazda S, Towart R. Nimodipine: a new calcium antagonistic drug with a preferential cerebrovascular action. Acta Neurochir (Wien). 1982; 63(1-4):259–265. PMID: 7102417.

24. Kirkpatrick PJ, Turner CL, Smith C, Hutchinson PJ, Murray GD. STASH Collaborators. Simvastatin in aneurysmal subarachnoid hemorrhage (STASH): a multicentre randomized phase 3 trial. Lancet Neurol. 2014; 7. 13(7):666–675. PMID: 24837690.
25. Kobayashi H, Ide H, Aradachi H, Arai Y, Handa Y, Kubora T. Histological studies of intracranial vessels in primates following transluminal angioplasty for vasospasm. J Neurosurg. 1993; 3. 78(3):481–486. PMID: 8433153.

26. Kuwayama A, Zervas NT, Shintani A, Pickren KS. Papaverine hydrochloride and experimental hemorrhagic cerebral arterial spasm. Stroke. 1972; Jan-Feb. 3(1):27–33. PMID: 5008302.

27. Kwak R, Niizuma H, Ohi T, Suzuki J. Angiographic study of cerebral vasospasm following rupture of intracranial aneurysms: Part 1. Time of the appearance. Surg Neurol. 1979; 4. 11(4):257–262. PMID: 441910.
28. Langham J, Goldfrad C, Teasdale G, Shaw D, Rowan K. Calcium channel blockers for acute traumatic brain injury. Cochrane Database Syst Rev. 2003; (4):CD000565. PMID: 14583925.

29. Lennihan L, Mayer SA, Fink ME, Beckford A, Paik MC, Zhang H, et al. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage: a randomized controlled trial. Stroke. 2000; 2. 31(2):383–391. PMID: 10657410.
30. Macdonald RL, Kassell NF, Mayer S, Ruefenacht D, Schmiedek P, Weidauer S, et al. Clazosentan toovercome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1) : randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008; 11. 39(11):3015–3021. PMID: 18688013.
31. Mindea SA, Yang BP, Bendok BR, Miller JW, Batjer HH. Endovascular treatment strategies for cerebral vasospasm. Neurosurg Focus. 2006; 9. 21(3):E13.

32. Murai Y, Kominami S, Kobayashi S, Mizunari T, Teramoto A. The long-term effects of transluminal balloonangioplasty for vasospasms after subarachnoid hemorrhage: analyses of cerebral blood flow and reactivity. Surg Neurol. 2005; 8. 64(2):122–126. discussion 127. PMID: 16051001.
33. Newell DW, Eskridge JM, Mayberg MR, Grady MS, Winn HR. Angioplasty for the treatment of symptomatic vasospasm following subarachnoid hemorrhage. J Neurosurg. 1989; 11. 71(5 Pt 1):654–660. PMID: 2530321.

34. Ogata M, Marshall BM, Lougheed WM. Observation on the effects of intrathecal papaverine in experimental vasospasm. J Neurosurg. 1973; 1. 38(1):20–25. PMID: 4629882.
35. O'Driscoll G, Green D, Taylor RR. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation. 1997; 3. 95(5):1126–1131. PMID: 9054840.
36. Peroutka SJ, Allen GS. Calcium channel antagonist binding sites labeled by 3H-nimodipine in human brain. J Neurosurg. 1983; 12. 59(6):933–937. PMID: 6631515.

37. Pisani A, Calabresi P, Tozzi A, D'Angelo V, Bernardi G. L-type Ca2+ channel blockers attenuate electrical changes and Ca2+ rise induced by oxygen/glucose deprivation in cortical neurons. Stroke. 1998; 1. 29(1):196–201. PMID: 9445351.
38. Rosenwasser RH, Armonda RA, Thomas JE, Benitez RP, Gannon PM, Harrop J. Therapeutic modalities for the management of cerebral vasospasm: timing of endovascular options. Neurosurgery. 1999; 5. 44(5):975–979. PMID: 10232530.

39. Sayama CM, Liu JK, Couldwell WT. Update on endovascular therapies for cerebral vasospasm induced by aneurysmal subarachnoid hemorrhage. Neurosurg Focus. 2006; 9. 21(3):E12.

40. Schmid-Elsaesser R, Kunz M, Zausinger S, Prueckner S, Briegel J, Stieger HJ. Intravenous magnesium versus nimodipine in the treatment of patients with aneurysmal subarachnoid hemorrhage: a randomized study. Neurosurgery. 2006; 6. 58(6):1054–1065. PMID: 16723884.

41. Schuknecht B. Endovascular treatment of cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl. 2005; 94:47–51. PMID: 16060240.

42. Shaw MD, Vermeulen M, Murray GD, Pickard JD, Bell BA, Teasdale GM. Efficacy and safety of the endothelinA/B receptor antagonist TAK-044 in treating subarachnoid hemorrhage: a report by the Steering Committee on behalf of the UK/Netherlands/Eire TAK-044 Subarachnoid Haemorrhage Study Group. J Neurosurg. 2000; 12. 93(6):992–997. PMID: 11117873.
43. Stippler M, Crago E, Levy EI, Kerr ME, Yonas H, Horowitz MB, et al. Magnesium infusion for vasospasm prophylaxis after subarachnoid hemorrhage. J Neurosurg. 2006; 11. 105(5):723–729. PMID: 17121134.

44. Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006; 1. 354(4):387–396. PMID: 16436770.

45. Su SH, Xu W, Hai J, Wu YF, Yu F. Effects of statins-use for patients with aneurysmal subarachnoid hemorrhage: a meta-analysis of randomized controlled trials. Sci Rep. 2014; 4. 4:4573. PMID: 24763190.

46. Vajkoczy P, Meyer B, Weidauer S, Raabe A. Thome C. Ringel F, et al. Clazosentan (AXV-034343), a selective endothelin A receptor antagonist, in the prevention of cerebral vasospasm following severe aneurysmal subarachnoid hemorrhage; results of a randomized, double-blind, placebo-controlled, multicenter phase IIa study. J Neurosurg. 2005; 7. 103(1):9–17. PMID: 16121967.
47. Veyna RS, Seyfried D, Burke DG, Zimmerman C, Mlynarek M, Nichols V, et al. Magnesium sulfate therapy after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002; 3. 96(3):510–514. PMID: 11883835.

48. Zubkov YN, Nikiforov BM, Shustin VA. Balloon catheter technique for dilatation of constricted cerebral arteries after aneurysmal SAH. Acta Neurochir (Wien). 1984; 70(1-2):65–79. PMID: 6234754.

Fig. 2
Aneurysm location in enrolled patients. Acom = anterior communicating artery; Pcom = posterior communicating artery; MCA = middle cerebral artery; ACA = anterior cerebral artery; ICA = internal carotid artery.
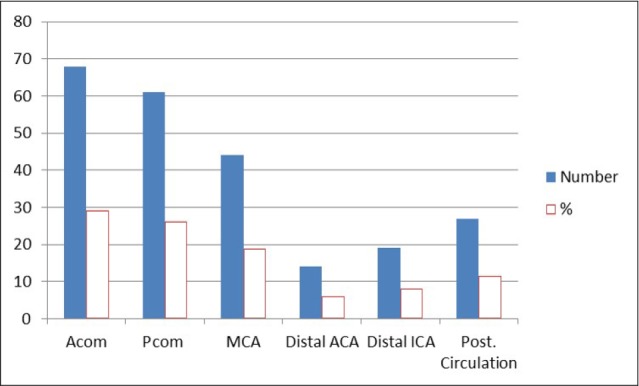
Fig. 3
Aneurysm location in symptomatic vasospasm. Acom = anterior communicating artery; Pcom = posterior communicating artery; MCA = middle cerebral artery; ACA = anterior cerebral artery; ICA = internal carotid artery.
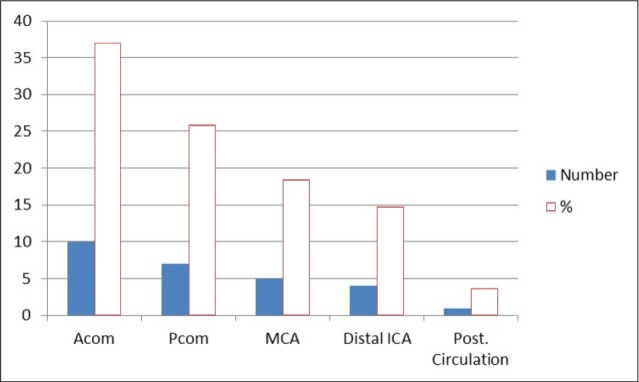
Table 1
Patients demographics and endovascular methods
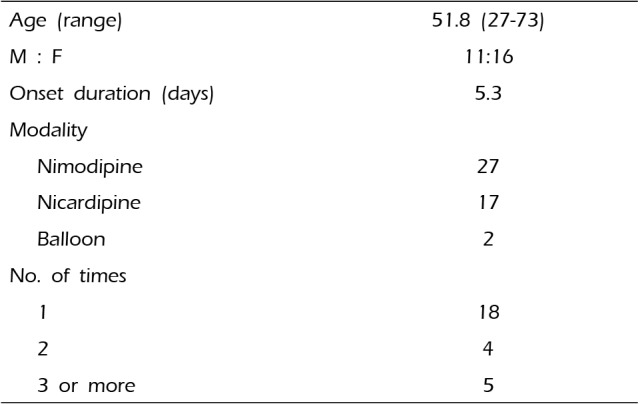
| Age (range) | 51.8 (27-73) |
| M : F | 11:16 |
| Onset duration (days) | 5.3 |
| Modality | |
| Nimodipine | 27 |
| Nicardipine | 17 |
| Balloon | 2 |
| No. of times | |
| 1 | 18 |
| 2 | 4 |
| 3 or more | 5 |
Table 2
Aneurysm location in enrolled patients
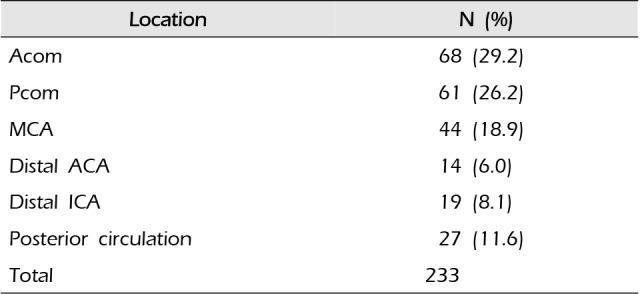
| Location | N (%) |
|---|---|
| Acom | 68 (29.2) |
| Pcom | 61 (26.2) |
| MCA | 44 (18.9) |
| Distal ACA | 14 (6.0) |
| Distal ICA | 19 (8.1) |
| Posterior circulation | 27 (11.6) |
| Total | 233 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download