Abstract
We report a case of dural arteriovenous fistula (DAVF) that showed spontaneous conversion of venous drainage pattern from Borden type II to type III within a four month period of follow-up. Upon admission, the patient presented with aggravated neurologic status and newly developed seizure. After admission, endovascular embolization was performed through the middle meningeal artery with Onyx®. Complete obliteration of dural arteriovenous shunt was confirmed by angiography, and the patient's clinical symptoms improved. Although most cases of DAVF show benign clinical course and conversion pattern, close follow-up is required to detect potential aggravation.
Dural arteriovenous fistula (DAVF), also called dural arteriovenous shunt or malformation, consists of acquired lesions within the dura mater.11)19) The etiology and natural history of these lesions remain unknown.18)19)23) However, the clinical presentations and assessed risk of the lesions have been correlated with the associated patterns of venous drainage. Cortical venous reflux is considered to be one important predictor of clinical course.7)8)18) Some authors have reported that some patients with DAVF show conversion of angiographic venous drainage pattern.4)11)12)18)19) Of these studies, most cases have exhibited chronological progression with spontaneous occlusion of the arteriovenous shunt. Only a small percentage of cases (about 1.7-4%) showed conversion to an aggressive lesion with symptomatic aggravation.1118)19) Therefore, in cases with worsening symptoms and neurological status, close clinical and radiological follow-up is particularly necessary.
We examined a case of spontaneous venous drainage pattern conversion from Borden type II to type III in a patient with DAVF. The patient presented with worsening neurological deficit and newly developed seizure within a four month period. The lesion was suspected to be caused by sinus thrombosis and rerouting of cortical venous reflux following venous infarction with mild hemorrhagic transformation.
A 64-year-old female patient was admitted to our institute with mild right hemiparesis, numbness on the right upper extremity, nausea, vomiting, and dizziness. Neurologic examination revealed grade IV+ right-side motor weakness. A magnetic resonance image (MRI) scan of the brain showed multiple small, round and tubular low-signal intensity regions on the left frontal lobe, a finding suggestive of vascular malformation. Initial digital subtraction angiography (DSA) identified a hypervascular lesion and arteriovenous shunting lesion on both external carotid artery (ECA) angiograms (Fig. 1). Both lesions suggestive of DAVF showed abnormal retrograde flow from the middle meningeal artery (MMA) and the superficial temporal artery into the superior sagittal sinus, including cortical venous reflux via the arteriovenous shunt (Borden classification II). We planned to treat the lesions with an endovascular approach because they were correlated with the patient's symptoms and showed cortical venous reflux. However, the patient and her family refused further treatment and requested hospital discharge.
At 4 months after hospital discharge, the patient was readmitted with repeated seizure that had been occurring for 3 weeks. The most recent seizure was a generalized tonic-clonic seizure with head turning and eyeball deviation to the right side; postictal confusion was also shown for 10 minutes. Brain MRI revealed subacute focal cerebral infarction with minimal hemorrhagic transformation on the left frontal lobe (Fig. 2). Antiepileptic drugs were initially loaded, and follow-up DSA was performed after 5 days when the patient's seizures were controlled by medication. DSA revealed a change of venous drainage pattern of the original lesions (Fig. 3). Abnormal venous reflux into the superior sagittal sinus was not present in the right ECA angiogram. Unexpectedly, only venous drainage directly into the subarachnoid cortical vein was observed, without contrast filling in the venous sinus in the left ECA angiogram (Borden classification III). Mild stenosis between the cortical vein and superior saggital sinus remained. We hypothesized that the hemiparesis and seizure arose from venous infarction with congestion of the left frontal lobe due to progression and rerouting of the cortical venous reflux after venous sinus thrombosis. We planned to treat the lesions with trans-arterial endovascular embolization with Onyx® (a nonadhesive liquid embolic agent; eV3 Neurovascular, Irvine, CA, USA).
Onyx® embolization was performed with successful obliteration of the arteriovenous shunt through the superselected left MMA in a single session (Fig. 4). Abnormal vascular structures, including cortical venous reflux, did not appear on any follow-up magnetic resonance angiography scans. The Glasgow outcome scale score was 2 when the patient was discharged, an improvement over the hemiparesis from her status at admission. Over a clinical follow-up period of 3 years, the patient did not experience any major problems.
Intracranial DAVF constitutes 10-15% of all intracranial vascular malformations, yet the natural course and pathogenesis of the lesions remain controversial.14)18)19)23) It is generally accepted that the presentation of DAVF is dictated by the location and pattern of venous drainage. Moreover, the cortical venous reflux pattern is an important predictor of clinical course.7)8)17) DAVFs with an aggressive course have characteristic angiographic features such as cortical or leptomeningeal venous drainage and an associated varix.17) In a meta-analysis, Awad et al.2) reported a hemorrhage rate of 88% and a severe neurologic deficit in 12% of 100 aggressive cases among 377 patients with DAVF. A previous study of Borden types II and III DAVFs reported an annual hemorrhage rate of 8.1% and an annual mortality rate of 10%.22) Another recent study reported an annual incidence of hemorrhage of 7.4% for patients presenting with an intracranial hemorrhage and 1.5% for those not presenting with hemorrhage.19) These discrepancies between the studies are likely due to demographic differences, different patient inclusion criteria, and publication bias. Knowledge about the natural disease course and classification based on the venous drainage pattern can inform decisions regarding the most appropriate treatment modality for these patients.
However, DAVF is a dynamic disease that can undergo spontaneous angiographic venous drainage pattern conversion. Kim et al.11) reported pattern conversion as assessed by angiography in 18 of 112 cases (16.1%). Among these cases, the conversion rate to an aggressive lesion was 4%; all of these cases were associated with occlusion of the ipsilateral draining vein. Moreover, several reports have described spontaneous closure of DAVF without any aggressive treatment.1)5)12) On the other hand, a few cases of conversion to aggressive lesions have been reported, and one study reported that benign AVFs have a 2% potential for angiographically verified conversion.18) Several theories have been suggested to explain the conversion of venous drainage pattern, including sinus thrombosis, change of sinus wall structure, and flow dynamics.2)12)16) Cerebral sinus thrombosis can cause spontaneous regression with symptom resolution and can also force retrograde venous reflux through the cortical veins, predisposing the patient to an aggressive clinical course.11)18)21) Thus, cerebral sinus thrombosis can promote the occlusion of venous outflow and result in dangerous rerouting. A histological study of DAVF suggested that microscopic thrombosis is always present, and that localizing thrombosis at the compartment or accessory mural channel of the drainage sinus may cause shunt occlusions.15) Stenosis and thrombosis of the venous outlets have been reported to forecast later worsening of DAVFs. Our case also showed stenosis of the venous outlets and ultimately showed spontaneous closure of the shunt into the superior saggital sinus and aggravated cortical venous reflux from Borden type II to type III.
Endovascular embolization has become the first line of treatment in patients with high-risk DAVFs, and surgery is considered only if endovascular treatments fail or are unfeasible. Trans-arterial approaches with embolic materials such as glue (NBCA: N-butyl cyanoacrylate), particles (PVA: polyvinyl alcohol), or Onyx® are performed to enable the embolic material to be pushed through the shunt into the proximal venous outlet. In recent studies, Onyx® has demonstrated multiple advantages over other materials, with reported occlusion rates of 62.5-80%.6)13)20) However, in cases of potentially dangerous occult ECA-internal carotid artery or vertebral artery anastomosis, development of ischemic cranial nerve palsies, or in fistulas with multiple feeders, a trans-venous approach is recommended.17) Trans-venous approaches can be performed with detachable coils and particles in the most proximal venous outlet and have been the treatment of choice, especially for carotid cavernous DAVFs.
The shunt locations and cortical venous reflux patterns may predict the clinical manifestations and drainage patterns of DAVFs. Superior saggital sinus DAVFs, as in our case, represent about 8-13% of all intracranial fistulae.10) These DAVFs are often accompanied by hemorrhage or progressive neurological deficits.24) These fistulae are characterized by bilateral supply from the MMA, ophthalmic artery, or posterior meningeal artery with critical venous drainage and require aggressive treatment.10) Despite the variety of treatment modalities available, trans-arterial embolization is recommended in superior saggital sinus DAVFs with adjuvant surgery or radiosurgery following failed embolization.3)9) In our case, we performed Onyx® embolization through the MMA, and successful obliteration was achieved in a single session. Due to the properties and increased predictability of Onyx®, more prolonged injections through single feeders like the MMA can be performed compared with other embolic materials.14) Moreover, the excellent flow control enables greater injection control, even when the vessel diameter is very small. With the newer and easier-to-navigate micro-catheters and the advent of Onyx®, trans-arterial embolization has become the accepted initial treatment for DAVF.
Although most cases of venous drainage pattern conversion in DAVF show benign clinical courses and do not develop symptoms, aggressive conversion sometimes occurs. Therefore, close clinical and radiological follow-up is needed to detect these lesions. Angiographic features should be the most important factor in deciding treatment modality. Our results indicate that endovascular embolization with Onyx® is a reliable treatment with acceptable safety and efficacy.
References
1. Al-Afif S, Nakamura M, Gotz F, Krauss JK. Spontaneous closure of a dural arteriovenous fistula. BMJ Case Rep. 2014; 7. 21:1–6.

2. Awad IA, Little JR, Akarawi WP, Ahl J. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg. 1990; 6. 72(6):839–850. PMID: 2140125.

3. Bertalanffy A, Dietrich W, Kitz K, Bavinzski G. Treatment of dural arteriovenous fistulae (dAVF's) at the superior sagittal sinus (SSS) using embolisation combined with micro- or radiosurgery. Minim Invasive Neurosurg. 2001; 12. 44(4):205–210. PMID: 11830779.

4. Chaudhary MY, Sachdev VP, Cho SH, Weitzner I Jr, Puljic S, Huang YP. Dural arteriovenous malformation of the major venous sinuses: an acquired lesion. AJNR Am J Neuroradiol. 1982; Jan-Feb. 3(1):13–19. PMID: 6800236.
5. Clarencon F, Biondi A, Sourour NA, Di Maria F, Iosif C, Nouet A, et al. Spontaneous closure of intracranial dural arteriovenous fistulas: a report of 3 cases. Clin Neurol Neurosurg. 2013; 7. 115(7):971–975. PMID: 23159510.
6. Cognard C, Januel AC, Silva NA Jr, Tall P. Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: new management using Onyx. AJNR Am J Neuroradiol. 2008; 2. 29(2):235–241. PMID: 17989374.

7. Davies MA, TerBrugge K, Willinsky R, Coyne T, Saleh J, Wallace MC. The validity of classification for the clinical presentation of intracranial dural arteriovenous fistulas. J Neurosurg. 1996; 11. 85(5):830–837. PMID: 8893721.

8. Fermand M, Reizine D, Melki JP, Riche MC, Merland JJ. Long term follow-up of 43 pure dural arteriovenous fistulae (AVF) of the lateral sinus. Neuroradiology. 1987; 29(4):348–353. PMID: 3627416.

9. Ghobrial GM, Marchan E, Nair AK, Dumont AS, Tjoumakaris SI, Gonzalez LF, et al. Dural arteriovenous fistulas: a review of the literature and a presentation of a single institution's experience. World Neurosurg. 2013; Jul-Aug. 80(1-2):94–102. PMID: 22381858.

10. Halbach VV, Higashida RT, Hieshima GB, Rosenblum M, Cahan L. Treatment of dural arteriovenous malformations involving the superior sagittal sinus. AJNR Am J Neuroradiol. 1988; Mar-Apr. 9(2):337–343. PMID: 3128082.
11. Kim DJ, terBrugge K, Krings T, Willinsky R, Wallace C. Spontaneous angiographic conversion of intracranial dural arteriovenous shunt: long-term follow-up in nontreated patients. Stroke. 2010; 7. 41(7):1489–1494. PMID: 20522815.
12. Luciani A, Houdart E, Mounayer C, Saint Maurice JP, Merland JJ. Spontaneous closure of dural arteriovenous fistulas: report of three cases and review of the literature. AJNR Am J Neuroradiol. 2001; 5. 22(5):992–996. PMID: 11337347.
13. Lv X, Jiang C, Zhang J, Li Y, Wu Z. Complications related to percutaneous transarterial embolization of intracranial dural arteriovenous fistulas in 40 patients. AJNR Am J Neuroradiol. 2009; 3. 30(3):462–468. PMID: 19131416.

14. Natarajan SK, Ghodke B, Kim LJ, Hallam DK, Britz GW, Sekhar LN. Multimodality treatment of intracranial dural arteriovenous fistulas in the Onyx era: a single center experience. World Neurosurg. 2010; 4. 73(4):365–379. PMID: 20849795.

15. Piske RL, Campos CM, Chaves JB, Abicalaf R, Dabus G, Batista LL, et al. Dural sinus compartment in dural arteriovenous shunts: a new angioarchitectural feature allowing superselective transvenous dural sinus occlusion treatment. AJNR Am J Neuroradiol. 2005; 8. 26(7):1715–1722. PMID: 16091520.
16. Saito A, Furuno Y, Nishimura S, Kamiyama H, Nishijima M. Spontaneous closure of transverse sinus dural arteriovenous fistula: case report. Neurol Med Chir (Tokyo). 008; 12. 48(12):564–568. PMID: 19106495.
17. Santillan A, Nanaszko M, Burkhardt JK, Patsalides A, Gobin YP, Riina HA. Endovascular management of intracranial dural arteriovenous fistulas: a review. Clin Neurol Neurosurg. 2013; 3. 115(3):241–251. PMID: 23287743.

18. Satomi J, van Dijk JM, Terbrugge KG, Willinsky RA, Wallace MC. Benign cranial dural arteriovenous fistulas: outcome of conservative management based on the natural history of the lesion. J Neurosurg. 2002; 10. 97(4):767–770. PMID: 12405361.

19. Soderman M, Pavic L, Edner G, Holmin S, Andersson T. Natural history of dural arteriovenous shunts. Stroke. 2008; 6. 39(6):1735–1739. PMID: 18388337.

20. Stiefel MF, Albuquerque FC, Park MS, Dashti SR, McDougall CG. Endovascular treatment of intracranial dural arteriovenous fistulae using Onyx: a case series. Neurosurgery. 2009; 12. 65(6 Suppl):132–139. discussion 139-40. PMID: 19934987.

21. Tsai LK, Jeng JS, Liu HM, Wang HJ, Yip PK. Intracranial dural arteriovenous fistulas with or without cerebral sinus thrombosis: analysis of 69 patients. J Neurol Neurosurg Psychiatry. 2004; 11. 75(11):1639–1641. PMID: 15489406.

22. van Dijk JM, terBrugge KG, Willinsky RA, Wallace MC. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke. 2002; 5. 33(5):1233–1236. PMID: 11988596.

23. Webb S, Hopkins LN. Intracranial dural arteriovenous fistulas: a treatment paradigm in flux. World Neurosurg. 2013; Jul-Aug. 80(1-2):47–49. PMID: 22548889.

24. Woo HH, Masaryk TJ, Rasmussen PA. Treatment of dural arteriovenous malformations and fistulae. Neurosurg Clin N Am. 2005; 4. 16(2):381–393. PMID: 15694169.

Fig. 1
Initial DSA of the patient (A : AP view of the right ECA angiography, B : AP view of the left ECA angiography) show hypervascular lesion and revealing dural arteriovenous fistula. (A) Right middle meningeal artery and superficial temporal artery supplied blood flow into superior saggital sinus via arteriovenous shunt. (B) Left middle meningeal artery supplied blood flow into superior saggital sinus via arteriovenous shunt. And there is stenosis at the proximal portion of superior saggital sinus. Cortical venous reflux is shown from superior saggital sinus to left cerebral hemisphere. DSA = digital subtraction angiography; AP = anteroposterior; ECA = external carotid artery.
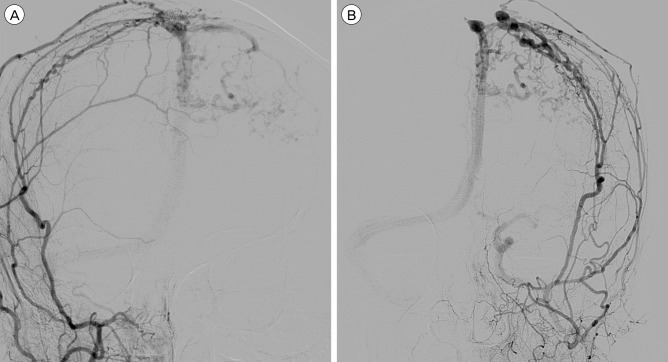
Fig. 2
Brain MRI performed when second admission after four months later from initial radiologic examination, show subacute focal infarction with minimal hemorrhagic transformation. (A) T2-weighted flair image show high signal intensity of left frontal lobe suggesting subacute cerebral infarction. (B) GRE image show multiple small low signal lesions suggesting minimal hemorrhagic transformation. (C) Mild gyral enhancement at the left frontal lobe is observed in gadolinium enhancement image. MRI = magnetic resonance image; GRE = gradient echo.
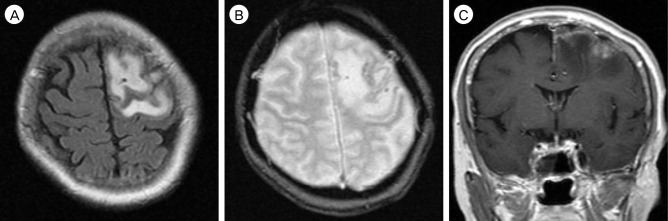
Fig. 3
Follow-up DSA after five months later from initial DSA revealing the change of venous drainage pattern. (A) Disappearance of arteriovenous shunting lesion in right ECA (external carotid artery) angiography. (B) Retrograde cortical venous reflux is shown without enhancement of superior saggital sinus in AP view of left ECA angiography. (C) There is stenotic lesion between the refluxed cortical vein and superior saggital sinus in lateral view of left ECA angiography. DSA = digital subtraction angiography; ECA = external carotid artery; AP = anteroposterior.
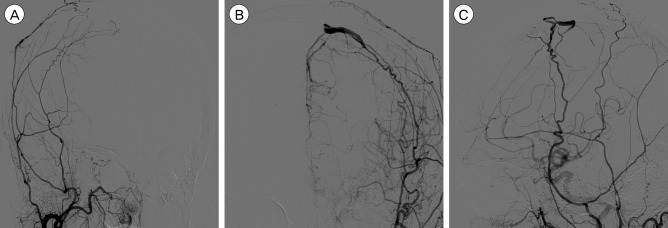
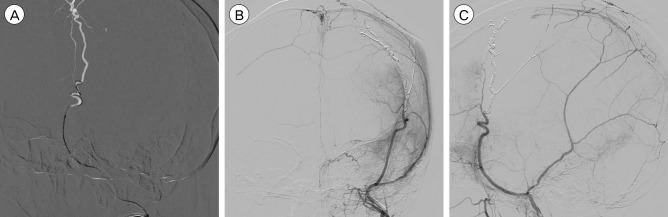
Fig. 4
After six days from second admission, Onyx embolization was performed. (A) Superselected left MMA with microcatheter and double micro-guidewire. AP view (B) and lateral view (C) after Onyx embolization show disappearance both of arteriovenous shunting lesion and cortical venous reflux. MMA = middle meningeal artery; AP = anteroposterior.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download