Abstract
This report describes a case of a serpentine fusiform aneurysm of the internal carotid artery in a patient who presented with visual disturbances. The serpentine aneurysm was treated successfully by coil trapping and occlusion of the parent artery, accompanied by balloon dilation. Nine months post-operatively, the patient's visual acuity had improved considerably.
Giant serpentine aneurysm was described in 1977 by Segel and McLaurin14) as a subcategory of giant aneurysms, distinct from the saccular variety. Patients with a large or giant aneurysm of the internal carotid artery (ICA) located in the paraclinoid area often present with cranial nerve symptoms. Cavernous ICA aneurysms can have a mass effect on the oculomotor, trochlear, or abducens nerve, resulting in internal or external ophthalmoplegia. Compression of the optic nerve results in impaired visual acuity and visual field defects. It is most likely associated with carotid ophthalmic or hypophyseal aneurysms, but rarely occurs with a cavernous ICA aneurysm. We report a patient with a serpentine cavernous ICA aneurysm who presented with visual symptoms that progressively improved after endovascular coil trapping.
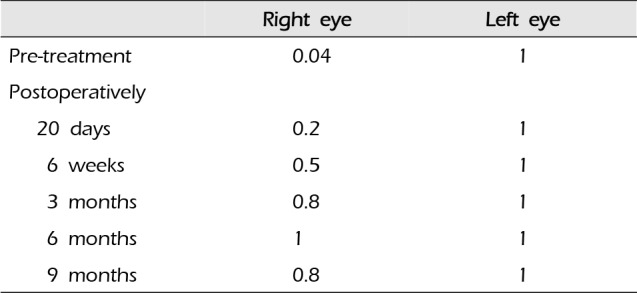
A 44-year-old woman presented with decreased right visual acuity of 1-month duration. Two weeks previously, she had complained of dry eye and was diagnosed with optic neuritis at a private clinic. She was then transferred to our hospital for further evaluation. The examination showed a decrease in her right visual acuity to 0.04 compared to 1.0 on the left side. The right visual field showed a 3/4 defect involving all but the right lateral upper quadrant. On magnetic resonance imaging (MRI) of the brain, a large aneurysm, 19 × 12 mm in size, well marginated, and oval-shaped, with a heterogeneous signal void was seen in the right parasellar region, probably in the cavernous sinus (Fig. 1A). Computed tomography angiography (CTA) showed fusiform dilatation of the right cavernous ICA, suggestive of a fusiform aneurysm with an approximate size of 30 × 20 mm (Fig. 1B). Fundus photography showed right optic nerve atrophy with a pale neural rim on the right optic disc (Fig. 2). The findings on transfemoral catheter angiography (TFCA) were similar to those obtained with MRI and CTA. The lesion identified on TFCA was 11.72 × 22.49 mm (Fig. 3). In treatment planning, the Matas test was performed using balloon dilation (Fig. 4A), followed by observation of the collateral flow during temporary occlusion of the parent artery. After 20 min, the collateral flow was deemed tolerable. The patient was then evaluated by single-photon emission computed tomography (SPECT). Because the results showed well-maintained vascular reserve (Fig. 4B), we chose to perform coil embolization of the large serpentine aneurysm with occlusion of the parent artery (Fig. 5). Postoperatively, the patient had no obvious neurological deficits. A second round of MRI and SPECT 24 h after the embolization showed no abnormalities. Nine months after coil embolization, the patient's visual field and visual acuity had improved considerably (Table 1, Fig. 6).
Reports on non-saccular cerebral aneurysms are complicated by the confusing terminology. Radiologically these aneurysms may be serpentine, whereas macroscopically they may be referred to as large fusiform aneurysms, and pathologically as dissecting, atherosclerotic, or dysplastic. This confusion about the exact nature of the aneurysm can be resolved by considering the natural history, clinical presentation, and treatment rationale. Patients with dissecting intracranial aneurysms typically present with subarachnoid hemorrhage and have a high incidence of early rebleeding.12) However, patients with large fusiform lesions present with a progressive mass effect and are at less risk of suffering hemorrhage.1)16)
The imaging features of large serpentine aneurysms are also characteristic.1)11) Computed tomography shows an extra-axial mass originating from the artery with intramural thrombosis. Magnetic resonance imaging shows a heterogeneous oval mass, indicative of thrombi of different ages, and a "flow-void" lesion with an enhanced rim. The cross-sectional angiographic image findings are typical.
Serpentine aneurysm is one of the most challenging vascular pathologies in terms of surgery. There are several reports of surgical (Hunterian) ligation, trapping, resection, and thrombectomy with and without vascular bypass.2)4)8)14)16) Surgical removal alone is a direct, attractive treatment. Several therapeutic options have been reported.1)3)9)13)14) However, the perioperative morbidity and mortality are 30-35%. One reason is the difficulty preserving the surrounding vessels during surgical dissection and excluding the aneurysm from the normal cerebral vasculature. Additionally, serpentine aneurysms are characterized by a relatively large volume of blood flow. In addition, the functionally distal areas of the brain are supplied by the channel within the serpentine aneurysm. Therefore, the goal of surgical treatment is the elimination of the blood flow to the aneurysm and maintenance of the blood flow to the distal cerebral territory.
Direct surgery has some limitations. First, the surgical dissection to expose the aneurysm may require aggressive retraction, resulting in brain injury. Second, adjacent important neurovascular structures can be compressed by the aneurysm and may adhere tightly to its surface. Third, the neck of a serpentine aneurysm can be difficult to identify in the surgical field. Some large serpentine aneurysms have been managed successfully using endovascular procedures intended to induce thrombosis and shrinkage of the mass.1) This is an indirect treatment strategy compared with surgical removal, but it has several advantages. An endovascular treatment does not require extensive retraction that can cause injury of the normal cerebral tissue due to microsurgical dissection. Furthermore, an endovascular treatment can be used to obtain clear angiographic images to visualize the serpentine vascular channels on the aneurysm surface.
Clinical improvement following endovascular occlusion of an aneurysm causing a mass effect has been reported in > 75% of cases.5)6)7)10)11) A dramatic decrease in aneurysm size is a common finding on MRI after successful endovascular occlusion.15)17) One report suggested that occlusion of the parent artery is more effective at decreasing the volume and relieving the mass effect than is endosaccular occlusion,17) which may result in the accumulation of embolic material in the aneurysm, thereby limiting shrinkage of the aneurysm. Nevertheless, endosaccular obliteration has been effective in reducing the mass effect.10) It has the advantage of preserving the normal brain and vascular structures.
Our messages in this case report are followings. In our patient, we did not clearly differentiate whether the pathogenesis of the visual disturbance involved embolism from the aneurysm itself or pulsatile compression by the aneurysm sac. However, our patient with a serpentine aneurysm presenting as a visual disturbance was treated by endovascular trapping. This was a better therapeutic choice than proximal occlusion because trapping can block both anterograde and retrograde flow, potentially reducing intra-aneurysmal pressure and promoting thrombogenesis. Careful studies were performed before trapping, using cross-compression and balloon occlusion to evaluate the cerebral hemodynamics. Because of the tolerable collateral flow, trapping was successful. However, in patients with poor collateral flow, extracranial-intracranial bypass surgery should be considered. Nonetheless, even in those cases, endovascular trapping is a useful option, particularly when performed in combination with bypass surgery.
References
1. Aletich VA, Debrun GM, Monsein LH, Nauta HJ, Spetzler RF. Giant serpentine aneurysms: a review and presentation of five cases. AJNR Am J Neuroradiol. 1995; 5. 16(5):1061–1072. PMID: 7639128.
2. Drake CG, Peerless SJ. Giant fusiform intracranial aneurysms: review of 120 patients treated surgically from 1965 to 1992. J Neurosurg. 1997; 8. 87(2):141–162. PMID: 9254076.

3. Greene KA, Anson JA, Spetzler RF. Giant serpentine middle cerebral artery aneurysm treated by extracranial-intracranial bypass. Case report. J Neurosurg. 1993; 6. 78(6):974–978. PMID: 8487082.
4. Haddad GF, Haddad FS. Cerebral giant serpentine aneurysm: case report and review of the literature. Neurosurgery. 1988; 7. 23(1):92–97. PMID: 3050586.

5. Halbach VV, Higashida RT, Dowd CF, Barnwell SL, Fraser KW, Smith TP, et al. The efficacy of endosaccular aneurysm occlusion in alleviating neurological deficits produced by mass effect. J Neurosurg. 1994; 4. 80(4):659–666. PMID: 8151344.

6. Higashida RT, Halbach VV, Dowd C, Barnwell SL, Dormandy B, Bell J, et al. Endovascular detachable balloon embolization therapy of cavernous carotid artery aneurysms: results in 87 cases. J Neurosurg. 1990; 6. 72(6):857–863. PMID: 2338569.

7. Larson JJ, Tew JM Jr, Tomsick TA, van Loveren HR. Treatment of aneurysms of the internal carotid artery by intravascular balloon occlusion: long-term follow-up of 58 patients. Neurosurgery. 1995; 1. 36(1):26–30. discussion 30. PMID: 7708164.

8. Lee KC, Joo JY, Lee KS, Shin YS. Recanalization of completely thrombosed giant aneurysm: case report. Surg Neurol. 1999; 1. 51(1):94–98. PMID: 9952130.

9. Lukin RR, Chambers AA, McLaurin R, Tew J Jr. Thrombosed giant middle cerebral aneurysms. Neuroradiology. 1975; 12. 10(3):125–129. PMID: 1207884.

10. Malisch TW, Guglielmi G, Vinuela F, Duckwiler G, Gobin YP, Martin NA, et al. Unruptured aneurysms presenting with mass effect symptoms: response to endosaccular treatment with Guglielmi detachable coils. Part I. Symptoms of cranial nerve dysfunction. J Neurosurg. 1998; 12. 89(6):956–961. PMID: 9833822.
11. Mawad ME, Klucznik RP. Giant serpentine aneurysms: radiographic features and endovascular treatment. AJNR Am J Neuroradiol. 1995; 5. 16(5):1053–1060. PMID: 7639127.
12. Mizutani T, Aruga T, Kirino T, Miki Y, Saito I, Tsuchida T. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery. 1995; 5. 36(5):905–911. discussion 912-3. PMID: 7791980.

13. Patel DV, Sherman IC, Hemmati M, Ferguson RJ. Giant serpentine intracranial aneurysm. Surg Neurol. 1981; 12. 16(6):402–407. PMID: 7330759.

14. Segal HD, McLaurin RL. Giant serpentine aneurysm. Report of two cases. J Neurosurg. 1977; 1. 46(1):115–120. PMID: 830809.
15. Strother CM, Eldevik P, Kikuchi Y, Graves V, Partington C, Merlis A. Thrombus formation and structure and the evolution of mass effect in intracranial aneurysms treated by balloon embolization: emphasis on MR findings. AJNR Am J Neuroradiol. 1989; Jul-Aug. 10(4):787–796. PMID: 2505506.
16. Tomasello F, Albanese V, Cioffi FA. Giant serpentine aneurysms: a separate entity. Surg Neurol. 1979; 11. 12(5):429–432. PMID: 515943.
17. Tsuura M, Terada T, Nakamura Y, Nakai K, Itakura T. Magnetic resonance signal intensity and volume changes after endovascular treatment of intracranial aneurysms causing mass effect. Neuroradiology. 1998; 3. 40(3):184–188. PMID: 9561526.

Fig. 1
(A) MRI shows a large thrombosed aneurysm 19 × 12 mm in size, well marginated, and oval-shaped, with a heterogeneous signal void in the right parasellar region, probably in the cavernous sinus. (B) CTA shows a fusiform dilatation of the right cavernous ICA, suggestive of a fusiform aneurysm approximately 30 × 20 mm in size. MRI = magnetic resonance imaging; CTA = computed tomography angiography; ICA = internal carotid artery.
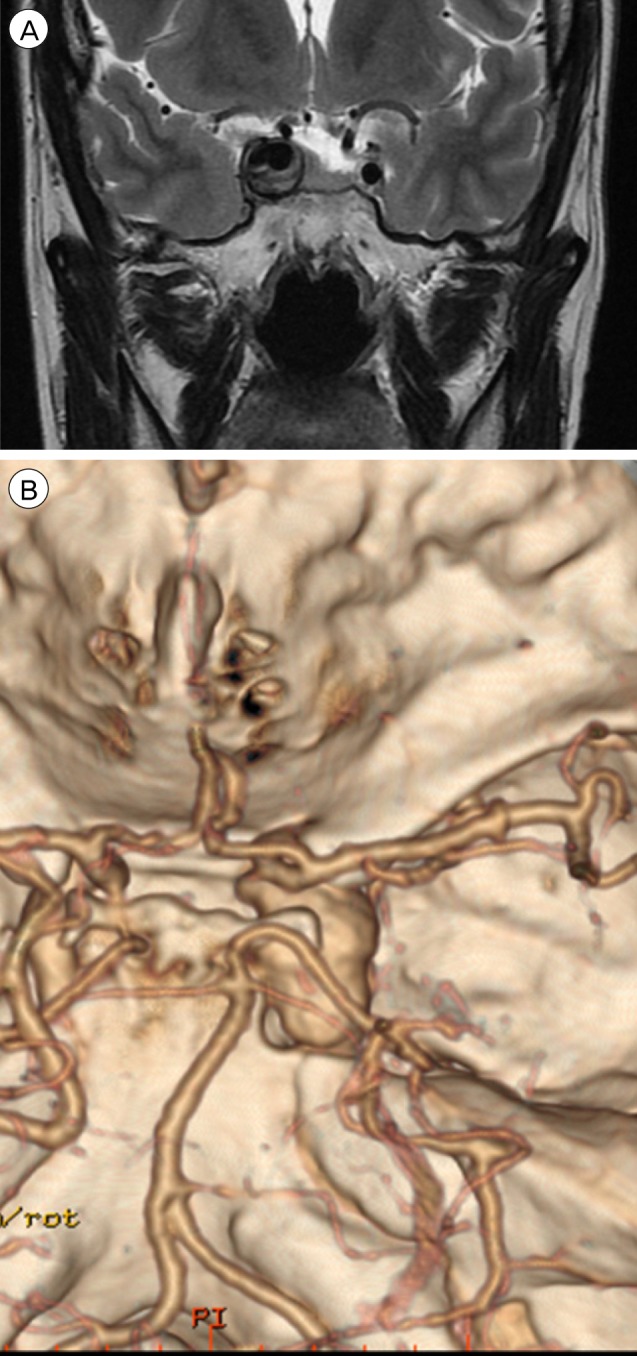
Fig. 2
Fundus photography shows right optic nerve atrophy, with a pale neural rim on the right optic disc.
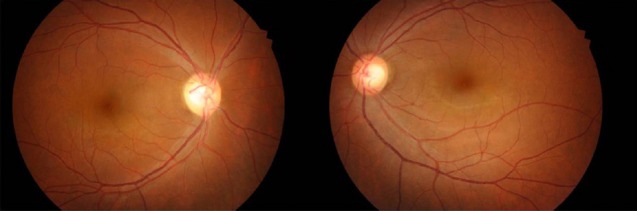
Fig. 3
3D angiography shows a large serpentine aneurysm of the right ICA 11.72 × 22.49 mm in size. 3D = three-dimensional; ICA = internal carotid artery.
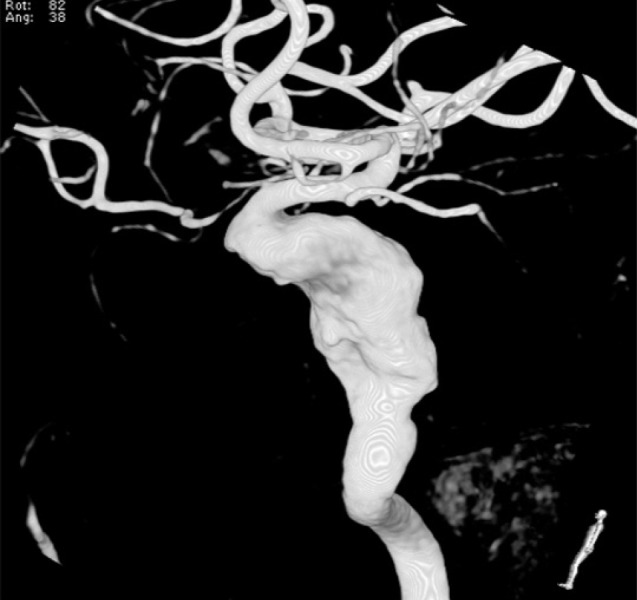
Fig. 4
(A, B) Matas test performed on the right ICA using balloon dilation of the parent artery followed by observation of the patient for 20 min. (C) SPECT shows the well-maintained vascular reserve. (D, E-1, E-2) Collateral flow from the left ICA & vertebrobasilar system was well visualized on preoperative cerebral angiography. ICA = internal carotid artery; SPECT = single-photon emission computed tomography.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download