Abstract
We report the case of a recurrent carotid cavernous fistula (CCF) originating from a giant cerebral aneurysm (GCA) after placement of a covered stent. A 47-year-old woman presented with sudden onset of severe headache, and left-sided exophthalmos and ptosis. Cerebral angiography revealed a CCF caused by rupture of a GCA in the cavernous segment of the left internal carotid artery. Two covered stents were placed at the neck of the aneurysm. The neurological symptoms improved at first, but were aggravated in the 6 months following the treatment. Contrast agent endoleak was seen in the distal area of the stent. Even though additional treatments were attempted via an endovascular approach, the CCF could not be cured. However, after trapping the aneurysm using coils and performing superficial temporal artery-middle cerebral artery bypass, the neurological symptoms improved. In cases of recurrent CCF originating from a GCA after placement of a covered stent, it is possible to treat the CCF by endovascular trapping and surgical bypass.
A carotid cavernous fistula (CCF) can be classified into direct or indirect types, or a combination of both. The direct type mainly results from trauma or rupture of a cavernous internal carotid artery (ICA) aneurysm. Cavernous ICA aneurysms account for 1.9-9.0% of intracranial aneurysms.13) About 1.5-9.0% of cavernous ICA aneurysms are complicated by a direct CCF.10)17) Furthermore, aneurysmal CCFs account for about 20% of direct CCFs.11) CCFs may produce a series of symptoms, including exophthalmos, conjunctival injection, bruit, and cranial nerve impairment. Moreover, drainage to the cortical veins may lead to intracranial hemorrhage,17) which requires careful treatment. At present, the first-line approach to treat CCF involves selecting an endovascular approach.6)9)11)17) Furthermore, covered stents are frequently used for treatment of direct CCFs.12)16)20) However, the first treatment attempt is not always successful. In this situation, a second treatment attempt (i.e., repeat endovascular treatment, surgical treatment, or a combination of both) should be considered.11)12) We report the case of a recurrent CCF originating from a giant cerebral aneurysm (GCA) after placement of a covered stent.
A 47-year-old woman presented with sudden severe headache, exophthalmos, and left-sided ptosis. The symptoms had first appeared 4 days earlier. In the neurological examination, medial gaze limitation in the left eye was observed, although light reflex was prompt. In brain computed tomography (CT) angiography, an enlarged ICA with the appearance of a giant aneurysm was identified in the left cavernous sinus. Cerebral angiography revealed a CCF caused by rupture of a GCA. The fistula began in the dome of the aneurysm, and had high blood flow. Venous reflux occurred through the superior ophthalmic vein, cerebral cortical vein, and pterygoid plexus (Fig. 1).
To determine the type of treatment, balloon test occlusion (BTO) was performed. Using a Scepter C dual-lumen balloon catheter (Microvention, Tustin, CA, USA), the aneurysm was temporarily trapped for 30 min, without any clinical change in the patient. During left ICA occlusion, technetium-99 m was injected via the venous root. After BTO, single-photon emission computed tomography (SPECT) was performed. In brain SPECT, decreased diffuse perfusion was seen in the left hemisphere. Considering these results, the patient was not considered suitable for ICA occlusion.
On the 4th day after admission, limitation of the left eye movement became aggravated, except for minimal lateral movement. For endovascular treatment, the patient was administered aspirin (100 mg) and clopidogrel (75 mg) from the time of admission. Five days later, the P2Y12 Reaction Units value was 331 and the Aspirin Reaction Units value was 551 in the VerifyNow System (Accriva Diagnostics, San Diego, CA, USA), indicating low responsiveness to both of these agents. Thus, endovascular treatment was delayed to the 9th day of admission, with addition of cilostazol 200 mg per day (i.e., triple antiplatelet therapy). Thereafter, we opted for treatment using an endovascular approach.
After gaining access to the left petrous ICA with a 7F Shuttle sheath (Cook, Bloomington, IN, USA) and a 6F Envoy guiding catheter (Cordis, Miami Lakes, FL, USA), a covered stent of 3.5 mm × 19 mm in size (Graftmaster RX; Abbott, IL, USA) was placed in the cavernous ICA between the anterior genu and the horizontal segment. After deployment of the stent, the aneurysm was still filled with contrast medium through the remnant neck of the aneurysm. Therefore, partially overlapping stenting, using another 4 mm × 19 mm covered stent, was performed proximal to the previous deployed stent. After balloon angioplasty, follow-up angiography showed that the aneurysm had disappeared (Fig. 2). After endovascular treatment, the neurological symptoms of the patient improved rapidly, except for mild chemosis. Three weeks after endovascular treatment, a VerifyNow test was performed during follow-up. The P2Y12 Reaction Units value was 280, which indicated resistance to clopidogrel, but the Aspirin Reaction Units value was 380, indicating responsiveness. Therefore, the patient was maintained on dual antiplatelet treatment with aspirin and cilostazol. There was no complication in the subsequent 6-months period.
However, after 6 months, the chemosis became aggravated. Therefore, brain CT angiography was performed, which revealed recurrence of the giant aneurysm and venous reflux. Cerebral angiography also confirmed recurrence of the CCF with the GCA (Fig. 3).
To locate the leakage point, several attempts of superselective angiography using an Echelon-10 microcatheter (eV3 Neurovascular, Inc., Irvine, CA, USA) were made. Contrast agent endoleak was seen in the distal area of the stent. Angioplasty was performed through the left ICA using a gateway balloon (Boston Scientific, San Leandro, CA, USA), but there was no interval change in the angiography. Therefore, we attempted aneurysm selection by means of a microcatheter, but failed. The microcatheter was then introduced into the left distal ICA via an A-com channel from the right ICA. After several attempts, we succeeded in selecting the aneurysm through the gap between the stent and ICA. Although we endeavored to pack the aneurysm with coils, it remained insufficiently packed. We had to finish the procedure with occlusion of the superior ophthalmic vein. After the procedure, the patient complained of a severe headache. After 1 week, we noted low density in the left temporal lobe on brain CT, which was thought to reflect a venous infarction (Fig. 4).
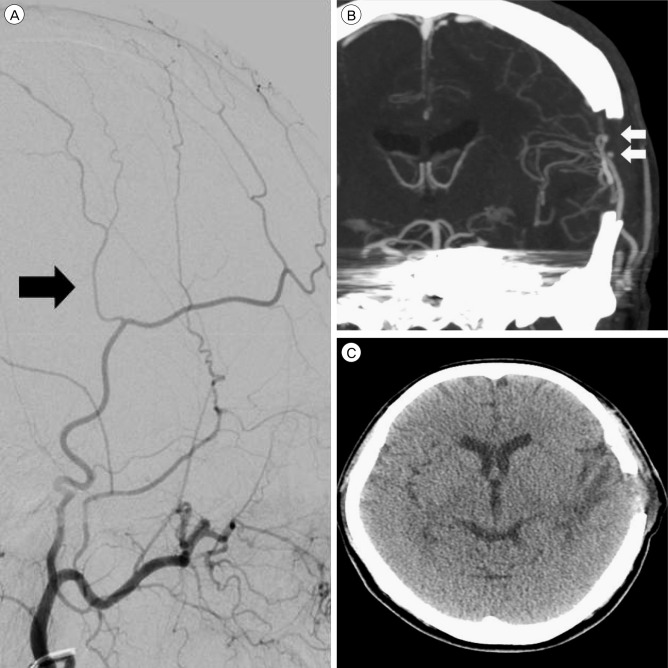
Ten days after the second endovascular treatment, a third endovascular treatment was performed. We attempted to approach the aneurysm via an A-Com and P-Com channel, and via the inferior petrosal sinus, but these approaches failed. Hence, as part of a planned internal trapping, the area from the distal part of the covered stent to the ophthalmic artery was temporally occluded using a Scepter C dual-lumen balloon catheter. After the distal part of the covered stent to the ophthalmic artery was occluded using the Scepter C balloon, angiography revealed choroidal blush and disappearance of the CCF. We then immediately performed internal trapping using a coil. After trapping, angiography revealed complete occlusion of the ICA from the region proximal to the stent to the region distal to the ophthalmic artery, aneurysm, and CCF (Fig. 5). Directly afterwards, we performed a superficial temporal artery middle cerebral artery bypass. Because of the small caliber of the parietal branch, a double-barrel bypass was performed. Thereafter, the patient's headache was relieved (Fig. 6). Although mild ptosis remained, the patient achieved a stable condition. During clinical follow-up for another 6 months, no neurological abnormality recurred.
GCAs frequently occur in the cavernous ICA. GCAs in the cavernous ICA usually have a benign natural course, but the rupture risk of a GCA is significantly higher in patients without thrombi or calcification.5)10)17) In the case presented here, the GCA was not accompanied by thrombi or calcification.
In ICA lesions, deconstructive treatment, such as trapping or proximal occlusion, through BTO (lasting 30 min) is usually considered safe. However, a good outcome after simple deconstruction cannot be ensured by observation of the neurological changes that occur during BTO.18) Therefore, treatment should be chosen in consideration of various conditions, such as delayed angiographic venous drainage, brain SPECT, etc.15)19)
Our patient's status was clinically acceptable during BTO. However, one should be aware that late complications such as ischemic symptoms could occur. Therefore, if ICA occlusion is necessary, additional bypass surgery may be helpful for preventing ischemic symptoms. However, our patient had cortical venous reflux. Because of brain swelling and venous engorgement, bypass surgery was not considered an option. Therefore, we planned STA-MCA bypass in case the first treatment attempt consisting of ICA occlusion via an endovascular approach failed.
Endovascular treatment is considered the first-line treatment for direct CCFs. Depending on the individual case, various materials, such as detachable balloons, coils, liquid material, and stents can be used.6)9)11)13)18)21) Currently, the covered stent is the preferred treatment option.1)8)12)16)20) However, spontaneous ICA occlusion and endoleaks frequently occur after placement of a covered stent.1)8)12)16)20) The covered stents in current use were designed for coronary arteries, and may be too stiff to navigate distal to the lesion and fit the cavernous siphon of the ICA appositely, frequently resulting in endoleaks. Archondakis et al. reported endoleaks in 3 of 8 cases.1) Wang et al.20) and Li et al.12) both reported 2 of 10 cases that were not resolved with balloon angioplasty.20 Many of these patients required further treatment, such as aneurysm coiling or ICA trapping.12)16)20)
In the case described here, the neck of the aneurysm included a wide area of the cavernous ICA, from the anterior genu to the horizontal segment. It was covered using two covered stents. Immediately after deployment of the stent, endoleak of contrast agent was still present. After balloon angioplasty, the endoleak disappeared. At that time, it appeared that the CCF had been treated appropriately using the covered stent. However, the CCF recurred during the follow-up period, due to endoleak. Wang et al. explained that recurrence of the CCF is related to spasm.20) However, in this case, the CCF recurred after 6 months, with relapse symptoms. We believe that recoiling the covered stent in the anterior genu of the cavernous ICA led to a gradual increase in the gap between the covered stent and the ICA. During endovascular retreatment, coil embolization of the GCA was attempted; however, this failed. Fortunately, there was collateral blood flow from the external carotid artery to the retina, so that endovascular trapping of the ICA with ophthalmic artery occlusion was possible.
Covered stents may not fit well in the carotid syphon. Therefore, in cases with aneurysms having a wide neck or located in the carotid syphon, it may not be possible to obtain good results with these stents. The experience from this case suggests the use of possibly more efficient alternative treatment options, such as stent-assisted coiling or a combination of covered stent and coiling. There is a need for development of new materials for use with covered stents for GCAs, taking into consideration the anatomical structure of the ICA.
During endovascular treatment, antiplatelet agent dual therapy consisting of aspirin and clopidogrel is commonly used to reduce thromboembolic events. Clopidogrel acts as a P2Y12 receptor inhibitor. Clopidogrel is activated in the body in two steps, and many factors, including genetic variation, influence these steps. Therefore, the degree of drug reaction may show individual differences. Insufficiency of P2Y12 receptor inhibition, despite usual doses, is defined as clopidogrel resistance or high on-clopidogrel platelet reactivity (HPR).2) In HPR patients, there is a tendency for frequent stent-related thromboembolic events during percutaneous coronary intervention.7)14) In the cardiology field, studies are researching the usefulness of drug reinforcement therapy. Nowadays, second generation P2Y12 inhibitors, such as prasugrel, are widely used. A similar situation may also occur during neurointervention.3)4) Compared with the typical stents used for coil embolization, covered stents may have a higher thromboembolic risk. In our case, the patient was thought to develop HPR. Changing the drugs to a second generation P2Y12 inhibitor or an antiplatelet agent with distinct characteristics may prevent thromboembolic events. Actually, these drugs are frequently used. However, there are currently no clear guidelines for interpretation of the results or for use of antiplatelet medication. In this case, the procedure was completed without any complications during or after treatment using triple antiplatelet therapy. Further studies on this issue are required.
References
1. Archondakis E, Pero G, Valvassori L, Boccardi E, Scialfa G. Angiographic follow-up of traumatic carotid cavernous fistulas treated with endovascular stent graft placement. AJNR Am J Neuroradiol. 2007; 2. 28(2):342–347. PMID: 17297009.
2. Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, et al. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010; 9. 56(12):919–933. PMID: 20828644.

3. Delgado Almandoz JE, Crandall BM, Scholz JM, Fease JL, Anderson RE, Kadkhodayan Y, et al. Last-recorded P2Y12 reaction units value is strongly associated with thromboembolic and hemorrhagic complications occurring up to 6 months after treatment in patients with cerebral aneurysms treated with the pipeline embolization device. AJNR Am J Neuroradiol. 2014; 1. 35(1):128–135. PMID: 23828107.

4. Delgado Almandoz JE, Kadkhodayan Y, Crandall BM, Scholz JM, Fease JL, Tubman DE. Variability in initial response to standard clopidogrel therapy, delayed conversion to clopidogrel hyper-response, and associated thromboembolic and hemorrhagic complications in patients undergoing endovascular treatment of unruptured cerebral aneurysms. J Neurointerv Surg. 2014; 12. 6(10):767–773. PMID: 24353331.

5. dos Santos ML, Spotti AR, dos Santos RM, Borges MA, Ferrari AF, Colli BO, et al. Giant intracranial aneurysms: Morphology and clinical presentation. Neurosurg Rev. 2013; 1. 36(1):117–122. discussion 122. PMID: 22791075.

6. Eddleman CS, Surdell D, Miller J, Shaibani A, Bendok BR. Endovascular management of a ruptured cavernous carotid artery aneurysm associated with a carotid cavernous fistula with an intracranial self-expanding microstent and hydrogel-coated coil embolization: Case report and review of the literature. Surg Neurol. 2007; 11. 68(5):562–567. discussion 567. PMID: 17961748.

7. Geisler T, Zurn C, Simonenko R, Rapin M, Kraibooj H, Kilias A, et al. Early but not late stent thrombosis is influenced by residual platelet aggregation in patients undergoing coronary interventions. Eur Heart J. 2010; 1. 31(1):59–66. PMID: 19812059.

8. Gomez F, Escobar W, Gomez AM, Gomez JF, Anaya CA. Treatment of carotid cavernous fistulas using covered stents: Midterm results in seven patients. AJNR Am J Neuroradiol. 2007; 10. 28(9):1762–1768. PMID: 17885249.

9. Kobayashi N, Miyachi S, Negoro M, Suzuki O, Hattori K, Kojima T, et al. Endovascular treatment strategy for direct carotid-cavernous fistulas resulting from rupture of intracavernous carotid aneurysms. AJNR Am J Neuroradiol. 2003; 10. 24(9):1789–1796. PMID: 14561604.
10. Kupersmith MJ, Hurst R, Berenstein A, Choi IS, Jafar J, Ransohoff J. The benign course of cavernous carotid artery aneurysms. J Neurosurg. 1992; 11. 77(5):690–693. PMID: 1403108.

11. Lewis AI, Tomsick TA, Tew JM Jr. Management of 100 consecutive direct carotid-cavernous fistulas: Results of treatment with detachable balloons. Neurosurgery. 1995; 2. 36(2):239–244. discussion 244-5. PMID: 7731502.
12. Li K, Cho YD, Kim KM, Kang HS, Kim JE, Han MH. Covered stents for the endovascular treatment of a direct carotid cavernous fistula : Single center experiences with 10 cases. J Korean Neurosurg Soc. 2015; 1. 57(1):12–18. PMID: 25674338.

13. Polin RS, Shaffrey ME, Jensen ME, Braden L, Ferguson RD, Dion JE, et al. Medical management in the endovascular treatment of carotid-cavernous aneurysms. J Neurosurg. 1996; 5. 84:755–761. PMID: 8622148.

14. Sibbing D, Morath T, Braun S, Stegherr J, Mehilli J, Vogt W, et al. Clopidogrel response status assessed with multiplate point-of-care analysis and the incidence and timing of stent thrombosis over six months following coronary stenting. Thromb Haemost. 2010; 1. 103(1):151–159. PMID: 20062919.

15. Sugawara Y, Kikuchi T, Ueda T, Nishizaki M, Nakata S, Mochizuki T, et al. Usefulness of brain SPECT to evaluate brain tolerance and hemodynamic changes during temporary balloon occlusion test and after permanent carotid occlusion. J Nucl Med. 2002; 12. 43(12):1616–1623. PMID: 12468510.
16. Tiewei Q, Ali A, Shaolei G, Feng L, Zhongsong S, Xuesong L, et al. Carotid cavernous fistulas treated by endovascular covered stent grafts with follow-up results. Br J Neurosurg. 2010; 8. 24(4):435–440. PMID: 20515263.

17. van Rooij WJ, Sluzewski M, Beute GN. Ruptured cavernous sinus aneurysms causing carotid cavernous fistula: Incidence, clinical presentation, treatment, and outcome. AJNR Am J Neuroradiol. 2006; 1. 27(1):185–189. PMID: 16418380.
18. van Rooij WJ, Sluzewski M, Metz NH, Nijssen PC, Wijnalda D, Rinkel GJ, et al. Carotid balloon occlusion for large and giant aneurysms: Evaluation of a new test occlusion protocol. Neurosurgery. 2000; 7. 47(1):116–121. discussion 122. PMID: 10917354.

19. van Rooij WJ, Sluzewski M, Slob MJ, Rinkel GJ. Predictive value of angiographic testing for tolerance to therapeutic occlusion of the carotid artery. AJNR Am J Neuroradiol. 2005; 1. 26(1):175–178. PMID: 15661722.
20. Wang C, Xie X, You C, Zhang C, Cheng M, He M, et al. Placement of covered stents for the treatment of direct carotid cavernous fistulas. AJNR Am J Neuroradiol. 2009; 8. 30(7):1342–1346. PMID: 19342540.

21. Wanke I, Doerfler A, Stolke D, Forsting M. Carotid cavernous fistula due to a ruptured intracavernous aneurysm of the internal carotid artery: treatment with selective endovascular occlusion of the aneurysm. J Neurol Neurosurg Psychiatry. 2001; 12. 71(6):784–787. PMID: 11723203.

Fig. 1
Brain computed tomography (CT) angiography and transfemoral cerebral angiography. (A) Axial image of brain CT reveals a well-enhanced lesion in the left cavernous sinus. (B) Three-dimensional (3D) CT angiography reveals a giant cerebral aneurysm in a cavernous internal carotid artery (ICA). (C) The location and shape of the aneurysm can be seen in a coronal source image on CT angiography (white arrows indicate the proximal and distal neck of the aneurysm). (D) Lateral view of the left ICA by digital subtraction angiography reveals venous reflux of arterial blood flow. The block arrow indicates venous reflux through the superior ophthalmic vein. Black and white arrowheads indicate venous reflux through the vein of Labbé and the straight sinus, respectively.
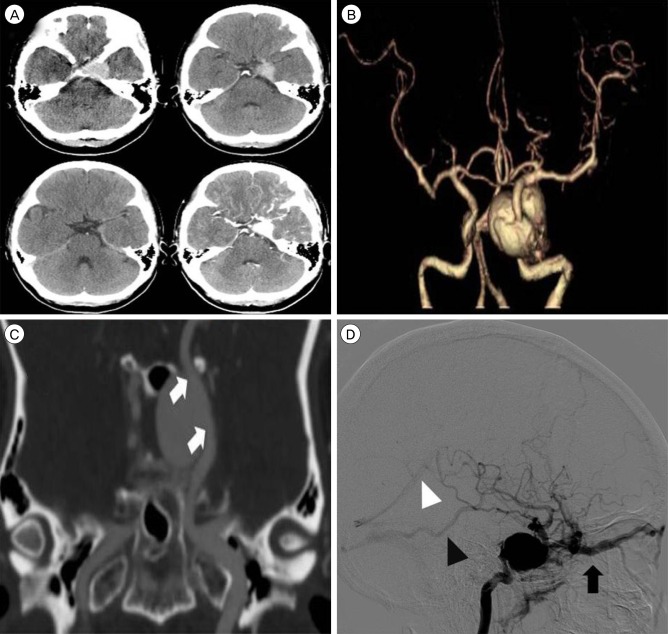
Fig. 2
(A) A 3.5 mm × 19 mm covered stent was placed in the cavernous internal carotid artery (ICA) between the anterior genu and the horizontal segment. Balloon angioplasty was performed, with inflation up to 20 atm (black arrowhead). (B) A 4 mm × 19 mm covered stent was placed in the cavernous ICA proximal to the previous deployed stent. Balloon angioplasty was performed, with inflation up to 24 atm (white arrowhead). (C, D) Follow-up left ICA angiography reveals disappearance of the aneurysm and the fistula. Anterograde blood flow to the left cerebral hemisphere can be clearly seen.
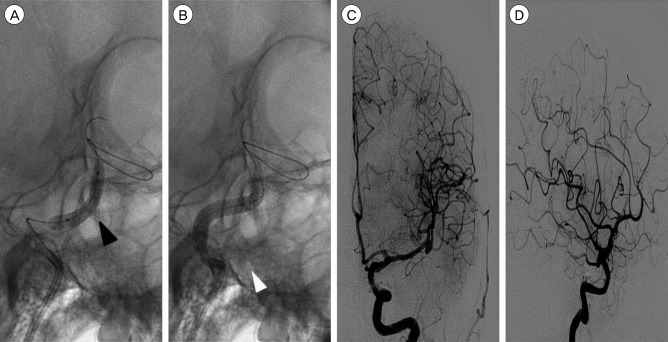
Fig. 3
(A) Computed tomography (CT) angiography after 6 months reveals recurrence of the giant aneurysm and carotid cavernous fistula. (B) In the lateral view on cerebral angiography, venous reflux can be seen to occur through the superior ophthalmic vein and cerebral cortical vein. A gap can be seen between the anterior genu of the ICA and the covered stent in a close-up view. ICA = internal carotid artery.
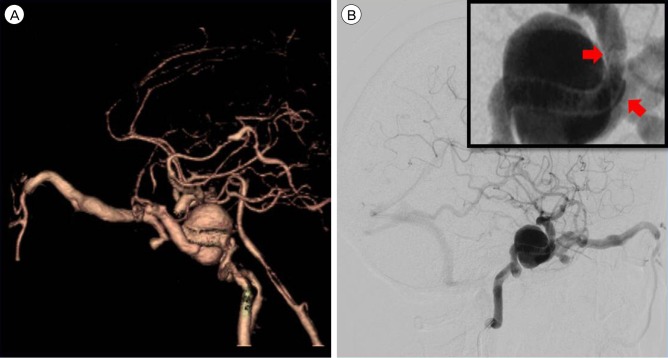
Fig. 4
Image taken during the second endovascular treatment. (A) The microcatheter can be seen entering the left distal internal carotid artery (ICA) via an A-Com channel, approaching from the right ICA, attempting to enter into the aneurysm through the gap between the stent and the ICA. (B) The aneurysm is packed using coils. However, the lower part of the aneurysm remained insufficiently packed. (C) In follow-up angiography, after coiling, reflux to the superior ophthalmic vein is found to be reduced. However, cortical venous reflux still exists. (D) On brain CT, low density and venous engorgement can be seen in the left temporal lobe. The asterisk indicates the infarct lesion. CT = computed tomography.
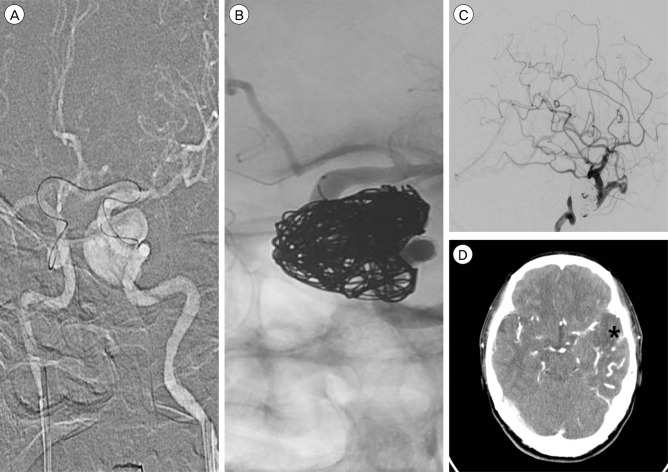
Fig. 5
Image obtained during the third endovascular treatment. (A) Cerebral angiography image obtained at 10 days after the previous treatment. Compared with the previous angiography, cortical venous reflux appeared to have increased. (B, C) After occluding the distal covered stent to the ophthalmic artery by means of a Scepter C balloon, angiography shows choroidal and disappearance of the CCF. (D) Angiography demonstrates complete occlusion of the ICA from the region proximal to the stent to the region distal to the ophthalmic artery. CCF = carotid cavernous fistula; ICA = internal carotid artery.
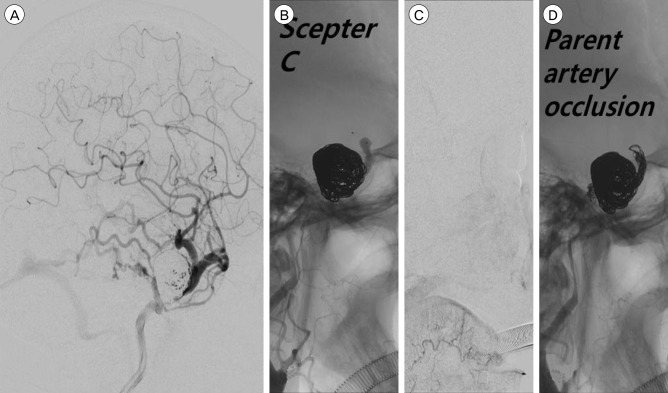
Fig. 6
Superficial temporal artery (STA) middle cerebral artery (MCA) bypass-related images. (A) Upon external carotid artery angiography, the caliber of the parietal branch seems smaller than that of the frontal branch (black arrow indicates parietal branch of the STA). (B) Coronal image taken during brain CTA reveals a patent double-barrel STA MCA bypass (white arrows indicate two anastomosis points). (C) A brain CT taken after 1 week reveals low density in the left temporal lobe that was thought to reflect the sequelae of a venous infarction. CTA = computered tomography angiography.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download