Abstract
Non-traumatic convexal subarachnoid hemorrhage (CSAH) is a comparatively infrequent with various vascular and nonvascular causes, it rarely occurs concomitant to acute ischemic stroke. We report a case of a 59-year-old woman, visited emergency room with right side subjective weakness spontaneously. Magnetic resonance diffusion-weighted images revealed an acute infarction of anterior cerebral arterial territory. Computed tomographic angiography showed a left frontal CSAH without any vascular lesions. And other laboratory studies were non-specific. We treated with dual antiplatelet drugs (cilostazole [Otsuka Pharmaceutical Co., Ltd. tokyo, Japan] and Aspirin [Bayer Pharma AG., Leverkusen, Germany]). She has done well for a follow-up period. (5 months) This case demonstrates the CSAH with acute infarction is rare but need to work up to identify the etiology and antiplatelet dugs are taken into account for treatments.
Non-traumatic convexal subarachnoid hemorrhage (CSAH) observed at the convexity of the brain is a relatively uncommon entity with various vascular and nonvascular causes.: cerebral venous thrombosis (CVT),1) reversible cerebral vasoconstriction syndrome (RCVS),4) vascular malformations, vasculitides,12) infectious aneurysms,10) Moyamoya disease or syndrome,22) severe carotid atherosclerosis,6) posterior reversible encephalopathy syndrome (PRES),24) cerebral amyloid angiopathy (CAA),5) and nonvascular disorders, such as primary and secondary brain neoplasms7) or abscess.18)
Cerebral infarcts in the territory of the anterior cerebral artery (ACA) are reported to comprise 0.5-3% of all ischemic strokes9)11) and few studies have specifically assessed the clinical characteristics of stroke patients with ACA infarction.2) We report a case of ACA cerebral infarction with spontaneous CSAH lead to stenosis of the ACA. It is a infrequent case, but is worth consideration that the management and evaluation of these patients.
A 59-year-old female patient visited emergency room with right side subjective weakness spontaneously. She had a clinical history of hypertension and other past medical history was unremarkable. There was no history of head trauma. Initial systolic and diastolic blood pressure was 140 mmHg / 80 mmHg. Routine laboratory finding is non-specific. She complained that right side subjective weakness and mild sensory numbness. However, The National Institute of Health Stroke Scale (NIHSS) was 0. Initial computed tomography angiography at one and half hours after symptom onset demonstrated subarachnoid hemorrhage (SAH) localized in the left frontal convexity and mild focal stenosis at both A2 segments. Also the Magnetic resonance diffusion-weighted images (DWI) revealed an acute infarction of anterior cerebral arterial territory (Fig. 1).
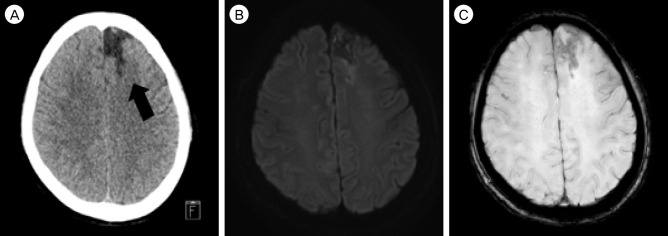
We could not find any vascular lesion on computed tomographic angiography (CTA) and magnetic resonance angiography (MRA) and treated with dual antiplatelet drugs (cilostazole [Otsuka Pharmaceutical Co., Ltd. tokyo, Japan] 50 mg bid/day plus aspirin [Bayer Pharma AG., Leverkusen, Germany] 100 mg) and hydration for 7 days. Other work up for evaluation of acute cerebral infarction was echocardiogram and holter monitoring. But there was no abnormal finding except a few APB on holter monitoring. The patient's neurological finding was improved on day 2. She has done well for a follow-up period and her Modified Rankin Scale was 0 at day 5. The follow-up evaluation was performed at out-patient clinic after 5 months. CTA shows that the SAH was washed out and encephalomalatic change in left ACA territory. MRI also reveals that no evidences of acute infarction in DWI and hemorrhagic transformation in susceptibility weighted imaging (SWI) (Fig. 2).
Although the prevalence of non-traumatic convexal SAH is reported to be 7.5% of all spontaneous SAH patients,13) little has been conscious of concerning the incidence of CSAH accompanying with acute infarction among many different etiologies of CSAH. Cuvinciuc et al. demostrated the image protocol to cover the wide spectum of entities potentially responsible for CSAH that brain CTA with a paired channel at both arterial and venous phases and MR imaging including GRE T2 sequences, FLAIR, DWI, MRA 3D TOF, contrast-enhanced venogram, and pre- and postgadoliniumT1-weighted imaging.3)
However, We evaluated the CTA and MR imaging cluding T2, DWI, SWI and 3D TOF MRA. these work-up tests were enough to distinguish the etiolgies of CSAH. CTA and MRA are useful to determine the vascular causes of CSAH such as vascular malformations, RCVS, vasulitides, high-grade stenosis, moyamoya disease, and septic aneurysms. In addition, MR imging is ascertainable diagnositic tools between dural and cortical CVT, and non-vascular causes such as, CAA, PRES, neoplasm, abscess and cavernoma.
In this case, ACA territory infarction seemed to occur hemodynamically because of stenotic lesion on A2 segment and hypoperfusion on brain SPECT with Tc-99 m HMPAO. Nakajima et al. assumed that the accompanying an infarction and non traumatic CSAH might demonstrate that hemodynamic insufficiency because of arterial stenosis or occlusion get to the critical point. For instance, after an acute infarction due to occlusive disease by the embolic or hemodynamic mechanism, ensuing dynamic changes of intracranial perfusion pressure might bring about the CSAH.14) Also Cuvinciuc et al. explained that the main mechanism of CSAH because of chronic arterial occlusive diseases is considered to be the rupture of dilated vulnerable compensatory pial vessels.3)
Antiplatelet treatment is suggested for patients who have suffered the infarction. A large randominzed controlled trial studied Clopidogrel and Aspirin in High-Risk Patients with Acute Nondisabling Cerebrovascular Events (CHANCE) was finished in china. The result revealed that antiplatelet double therapy with clopidogrel plus aspirin was better than aspirin alone for reducing the risk of recurrent stroke and not increase the risk of bleeding among patients with high risk transient ischemic attack (TIA) or minor infarction23) Due to our patient's acute hemorrhage on the frontal convexity, we were concerned the hemorrhagic transformation and rebleeding because dual antiplatelet therapy (clopidogrel plus aspirin) was associated with a significant trend to increase moderate bleeding.17) So we treated with cilostazol plus aspirin successfully. The patient's symptom was improved and there was no evidence of rebleeding on following studies. Tan et al. reported that cilostazol, alone or with aspirin, decrease recurrence of ischemic stroke significantly, poststroke intracranial hemorrhage, and extracranial bleeding in patients with a prior ischemic stroke as compared with other antiplatelet treatments.21) Cilostazol, a selective inhibitor of cyclic nucleotidephosphodiesterase 3, increases activated intracellular cyclic adenosine monophosphate (cAMP) concentrations and thus inhibits platelet aggregation.19) Cilostazol is a known direct arterial vasodilator,15) and has antiatherosclerotic effect,8) which can strengthen the endothelial barrier20) and additionally may play a role in neuroprotection.16)
Acute infarction with spontaneous convexal SAH is rare but it is worth to work up to identify the etiology of CSAH using the CTA or MRA and MR image including T2, DWI, SWI. And we suggest that dual antiplatelet dugs (cilostazole and aspirin) are taken into account for treatments that improved the neurologic symptoms and reduced the concerns about stroke recurrence and rebleeding.
References
1. Arévalo-Lorido JC, Carretero-Gómez J. Cerebral venous thrombosis with subarachnoid hemorrhage: a case report. Clin Med Res. 2015; 3. 13(1):40–43. PMID: 25380613.
2. Arboix A, García-Eroles L, Sellarés N, Raga A, Oliveres M, Massons J. Infarction in the territory of the anterior cerebral artery: clinical study of 51 patients. BMC Neurol. 2009; 7. 9:30. PMID: 19589132.

3. Cuvinciuc V, Viguier A, Calviere L, Raposo N, Larrue V, Cognard C, et al. Isolated acute nontraumatic cortical subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2010; 9. 31(8):1355–1362. PMID: 20093311.

4. Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007; 12. 130(Pt 12):3091–3101. PMID: 18025032.

5. Finelli PF. Cerebral amyloid angiopathy as cause of convexity SAH in elderly. Neurologist. 2010; 1. 16(1):37–40. PMID: 20065795.

6. Geraldes R, Santos C, Canhão P. Atraumatic localized convexity subarachnoid hemorrhage associated with acute carotid artery occlusion. Eur J Neurol. 2011; 2. 18(2):e28–e29. PMID: 20868466.

7. Hentschel S, Toyota B. Intracranial malignant glioma presenting as subarachnoid hemorrhage. Can J Neurol Sci. 2003; 2. 30(1):63–66. PMID: 12619787.

8. Ito H, Uehara K, Matsumoto Y, Hashimoto A, Nagano C, Niimi M, et al. Cilostazol inhibits accumulation of triglyceride in aorta and platelet aggregation in cholesterol-fed rabbits. PLoS One. 2012; 7(6):e39374. PMID: 22761774.

9. Kang SY, Kim JS. Anterior cerebral artery infarction: stroke mechanism and clinical-imaging study in 100 patients. Neurology. 2008; 6. 70(24 Pt 2):2386–2393. PMID: 18541871.

10. Kannoth S, Iyer R, Thomas SV, Furtado SV, Rajesh BJ, Kesavadas C, et al. Intracranial infectious aneurysm: presentation, management and outcome. J Neurol Sci. 2007; 5. 256(1-2):3–9. PMID: 17360002.

11. Kazui S, Sawada T, Naritomi H, Kuriyama Y, Yamaguchi T. Angiographic evaluation of brain infarction limited to the anterior cerebral artery territory. Stroke. 1993; 4. 24(4):549–553. PMID: 8465361.

12. Kumar R, Wijdicks EF, Brown RD Jr, Parisi JE, Hammond CA. Isolated angiitis of the CNS presenting as subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1997; 6. 62(6):649–651. PMID: 9219758.

13. Kumar S, Goddeau RP Jr, Selim MH, Thomas A, Schlaug G, Alhazzani A, et al. Atraumatic convexal subarachnoid hemorrhage: clinical presentation, imaging patterns, and etiologies. Neurology. 2010; 3. 74(11):893–899. PMID: 20231664.
14. Nakajima M, Inatomi Y, Yonehara T, Hirano T, Ando Y. Nontraumatic convexal subarachnoid hemorrhage concomitant with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014; 7. 23(6):1564–1570. PMID: 24630829.

15. Nakamura K, Ikomi F, Ohhashi T. Cilostazol, an inhibitor of type 3 phosphodiesterase, produces endothelium-independent vasodilation in pressurized rabbit cerebral penetrating arterioles. J Vasc Res. 2006; 43(1):86–94. PMID: 16286783.

16. Nonaka Y, Tsuruma K, Shimazawa M, Yoshimura S, Iwama T, Hara H. Cilostazol protects against hemorrhagic transformation in mice transient focal cerebral ischemia-induced brain damage. Neurosci Lett. 2009; 3. 452(2):156–161. PMID: 19383431.

17. O'Donnell MJ, Hankey GJ, Eikelboom JW. Antiplatelet therapy for secondary prevention of noncardioembolic ischemic stroke: a critical review. Stroke. 2008; 5. 39(5):1638–1646. PMID: 18369175.
18. Rhode V, van Oosterhout A, Mull M, Gilsbach JM. Subarachnoid haemorrhage as initial symptom of multiple brain abscesses. Acta Neurochir (Wien). 2000; 142(2):205–208. PMID: 10795896.
19. Sudo T, Tachibana K, Toga K, Tochizawa S, Inoue Y, Kimura Y, et al. Potent effects of novel anti-platelet aggregatory cilostamide analogues on recombinant cyclic nucleotide phosphodiesterase isozyme activity. Biochem Pharmacol. 2000; 2. 59(4):347–356. PMID: 10644042.

20. Sugiura Y, Morikawa T, Takenouchi T, Suematsu M, Kajimura M. Cilostazol strengthens the endothelial barrier of postcapillary venules from the rat mesentery in situ. Phlebology. 2014; 10. 29(9):594–599. PMID: 23858026.

21. Tan L, Margaret B, Zhang JH, Hu R, Yin Y, Cao L, et al. Efficacy and Safety of Cilostazol Therapy in Ischemic Stroke: A Meta-analysis. J Stroke Cerebrovasc Dis. 2015; 5. 24(5):930–938. PMID: 25804574.

22. Wan M, Han C, Xian P, Yang WZ, Li DS, Duan L. Moyamoya disease presenting with subarachnoid hemorrhage: Clinical features and neuroimaging of a case series. Br J Neurosurg. 2015; 29(6):804–810. PMID: 26313681.

23. Wang Y, Pan Y, Zhao X, Li H, Wang D, Johnston SC, et al. Clopidogrel With Aspirin in Acute Minor Stroke or Transient Ischemic Attack (CHANCE) Trial: One-Year Outcomes. Circulation. 2015; 7. 132(1):40–46. PMID: 25957224.
24. Yoon SD, Cho BM, Oh SM, Park SH, Jang IB, Lee JY. Clinical and radiological spectrum of posterior reversible encephalopathy syndrome. J Cerebrovasc Endovasc Neurosurg. 2013; 9. 15(3):206–213. PMID: 24167801.

Fig. 1
(A) Computed tomography scan of the patient showing subarachnoid hemorrhage (black arrow) on left frontal convexity. (B) CT angiography shows that both A2 segments were mild focal stenosis (white arrow). (C) Diffusion-weighted images shows a high intensity area in the territory of left anterior cerebral artery. (D) Brain SPECT with Tc-99 m HMPAO shows a small area of decreased perfusion at left frontal area. CT = computed thmography; SPECT = single-photon emission computed tomography; HMPAO = hexamethylpropylene amine oxime; Lt = left; Rt = Right.
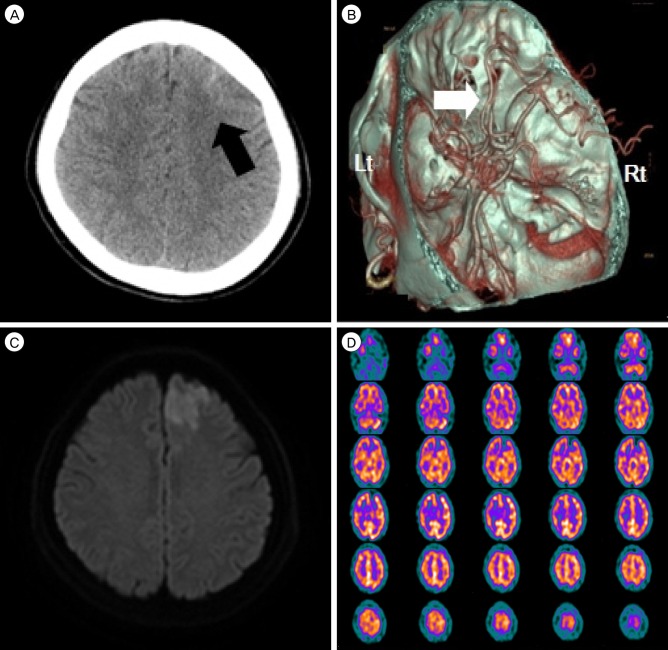
Fig. 2
(A) Computed tomography shows that low intensity of previous ACA infarction territory (black arrow) and no high intensity presenting SAH on left frontal convexity. (B) and (C) there is no evidence of acute infarction in DWI and hemorrhagic transformation in SWI. ACA = anterior cerebral artery; SAH = subarachnoid hemorrhage; DWI = diffusion-weighted images; SWI = susceptibility weighted imaging.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download