Abstract
Objective
We evaluated the feasibility of angiographic computed tomography (ACT) for visualizing stent material in patients who underwent intracranial or extracranial stent placement to treat atherosclerotic lesions or stent assisted coil embolization.
Materials and Methods
We performed intrarterial and intravenous ACT on biplane angiography system equipped with flat panel detectors (Axiom Arits dBA; Siemens Medical Solutions, Forchheim, Germany). Vistipaque 320 was injected for contrast medium, total 150 mL at flow rate of 5 mL/s through artery and 77 mL at flow rate of 3.5 mL/s through vein.
Angioplasty and stenting is a therapeutic option in symptomatic intracranial atherosclerotic disease. Also stent assisted aneurysm coil embolization is well established in wide necked aneurysm therapy. So we need exact evaluations that can assess stent positioning, adaptation of stent struts to the vessel wall or to exclude in-stent restenosis (ISR). angiographic computed tomography (ACT) is a new technique that provides a highest spatial resolution and high contrast resolution derived from a rotational acquisition of a c-arm-mounted flat-panel detector.4) We demonstrated the feasibility and diagnostic value of intrarterial (IA) and intravenous (IV) -ACT for pre or postprocedure imaging for stent angioplasty and stent assisted coil embolization of cerebral aneurysm.
Conventional digital subtraction angiography (DSA) and ACT were performed on biplane angiography system equipped with flat panel detectors (Axiom Arits dBA ; Siemens Medical Solutions, Forchheim, Germany). The parameters for acquisition of rotational datasets (20s-1k protocol): 20 seconds rotation, 538 projections, 220° total angle, no zoom (detector size 30 × 40 cm), CTDIw approximately 22 mGy (manufacturer's information). Post processing of the image data to a volume dataset was performed on a Leonardo workstation (DynaCT, InSpace 3D software, Siemens, Erlangen, Germany). The software includes the application of system-specific filter algorithms in order to correct for beam hardening, radiation scatter, truncated projections and ring artifacts. Post processing resulted in volume datasets each defined by a batch of about 400 slices in a 512 × 512 matrix. The contrast medium used in the IA-ACT examination was Visipaque320 (GE healthcare, carrigtohill, Ireland) The contrast medium was injected to a volume of 150 mL (contrast 100 mL with Saline 50 mL mixed) at a flow rate of 5 mL/s, and the start delay for rotational acquisition was 20 seconds(intracranial) or 15 seconds(VAO, CC-IC). The contrast medium was injected for IV-ACT to a volume of 77 mL into a artery at a flow rate of 3.5ml/s, and the start delay for rotational acquisition was 2 seconds.
A 53-year-old female was treated because of symptomatic 85% stenosis at the cervical internal carotid artery (ICA). A long segment of irregular atherosclerotic plaque was seen. We confirmed this lesion by IA-ACT. After a protective device (Angiogurd RX; Cordis) was placed in the distal cervical segment of the ICA, 8 × 40 mm device (Precise; Cordis) stent was applicated at the lesion. Because significant residual stenosis was present immediately after stenting, post-dilation of the stent was performed by using a 6 × 30 mm balloon. After the ballooning of the stent, residual stenosis was dilated about 50%. ACT had been performed at the end of the procedure. ACT showed incomplete stent deploying because extensive calcification in the carotid wall was compressing the segment of the stent. The extent of calcification in the circumference of the arterial wall was more clearly revealed in axial view. This extensive wall calcification explained why the stent could not be fully deployed over its entire length (Fig. 1).
A 66-year-old man presented with recurrent left hemiparesis. Diffusion magnetic resonance imaging (MRI) of the brain showed a minimal acute infarction in the basal ganglia (BG) and periventricular white matter (PVWM) on the left. Cerebral angiography showed severe stenosis (85%) of the M1 segment of the right middle cerebral artery (MCA).
Placement of a 2.5 × 8 mm balloon-expandable stent (Endeavor; Medtronic scientific, Roncadelle (BS), Italy) into the right M1 segment was successfully performed, with completely reestablishing the lumen and restoring normal flow. ACT was performed at the end of the procedure. Although the stent is not completely identified on DSA and is only slightly more visible on nonsubtracted views, ACT can detect the stent completely in every plane. The axial view demonstrated full and uniform deployment of the stent over its entire length. This finding could not be determined from the DSA. Follow up IV-ACT was performed after 3month later via out patient clinic. It showed no ISR and clear stent strut. (Fig. 2).
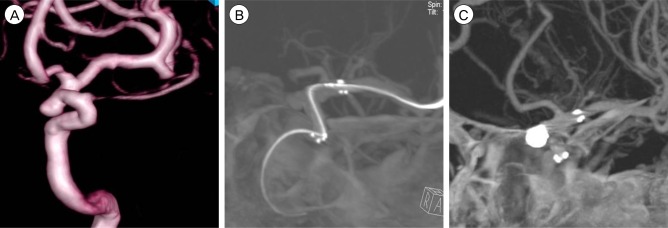
A 48-year-old man visited our hospital due to headache. CT angiography (CTA) showed unruptured aneurysm on paraclinoid ICA. Three dimensional (3D) DSA was performed and wide neck based aneurysm was confirmed. Stent assisted coil embolization was done under general anesthesia. Initially Self expandable stent 4.5 × 20 mm (Neuroform3 stent ; ~~~) was placed to entirely cover Neuroform3 neck. After stenting, IA-ACT was performed just before coil embolization in order to ensure perfect stent positioning. Total of 5 platinum microcoil were inserted in the paraclinoid aneurysm, which completely occluded the lesion. ACT performed after coil embolization allows to define the exclusion of stent strut movement or coil protrusion with minimal beam-hardening artifacts (Fig. 3).
However, introduction of small, low profile, high flexibility and excellent tractability stents makes available for wide use of stent for intracranial, extracranial atherosclerotic disease and stent assisted aneurysm coiling, resulting restenosis rates ranging from 8% to 30%.8)11) Also complications of stent assisted coil embolization such as incomplete stent deployment, stent migration, coil dislocation, and in-stent stenosis during the procedure or the follow period are still yet to be resolved. Therefore, pre-, intra- and post-procedural imaging studies, which can help to visualize the exact position of the stent and to identify significant ISR, are very crucial.
Currently DSA is the gold standard diagnostic tool. However, it still carries a 0.5% to 0.8% risk of permanent neurological impairment.5)15) In addition, DSA is difficult to be done in outpatient clinic and for patients with poor compliance, and it is also not a good imaging tool for follow-up (f/u) examination. Consequently, these problems force us to use other evaluation technique, such as brain CTA, Doppler sonography and plane x-ray.
Brain CTA can be used to assess vascular patency after stents. However, in vitro studies have demonstrated that the degree of ISR tends to be overestimated.12)
Duplex sonography also plays an important role in the pre- and post-procedure, especially in carotid stenosis, but the actual accuracy is dependent on the examiner.
Fluoroscopic and plain radiography can also be used, but these modalities are used only in cases where self expandable stent was applied. This type of stent is visible only at the proximal and the distal end where the radiopaque markers are located.
Because of these problems of previous evaluation tools, many additional imaging techniques are introduced nowadays, including ACT.
Synonyms of ACT are Flat Panel Angiographic Tomography, Flat Panel Detector Angiographic CT or system, Flat-Panel CT, Angiographic C-arm Tomography, Cone beam C-arm system, Dyna CT (Siemens), XPER CT (Philips, Amsterdam, The Netherlands).
Benefits of ACT are as follows : 1) Examinations can be performed on an outpatient basis 2) the risk of neurological complications is low 3) the examination can be performed quickly, in a time similar to that required for a CTA 4) the radiation exposure for the patient from a rotational acquisition is lower than that from a CTA examination (manufacturer's information 22 mGy vs. 50 mGy) 5) the patients do not have to be moved to CT scanner in order to confirm the position of the stent or coil during the procedure or immediately after the procedure.10)
As shown previously, we demonstrated that IA and IV ACT can be used as another diagnostic option for stents during procedure and the follow-up period, because it can reliably depict the lumen of small-vessel stents with high spatial resolution.6) Utilizing ACT imaging may enable adequate visualization of the stent position and especially the stent struts.2)3) All intracranial vessels can be viewed simultaneously in high-quality images, while CT-like images of the brain parenchyma are provided as well.
However, limitations arise with artifacts, including movement artifact, and with calibration problems that deteriorate image quality. The patient has to lie nearly motionless for about 20 seconds, requiring a high level of compliance, which is not always feasible in patients suffering from cerebrovascular disease.4)
Another limitation is that the contrast differentiation is inferior in areas of low radiographic contrast compared to conventional CT imaging. Also, reconstruction still requires 5 to 10 minutes, and the DynaCT procedure has not been accepted as a CT scan modality under the insurance system as it is still considered as fluororadiography.
Meanwhile, stent-assisted aneurysm coiling is well established in clinical routine.1)7)9)13)14) However, fluoroscopic and plain radiographic visibility of the stent struts is especially limited by the low profile of these highly flexible devices, with only the proximal and distal radiopaque stent markers visible. This may render it difficult to exactly assess stent position and adaptation of stent struts to the vessel wall.3)
ACT uses rotational, C-arm mounted flat panel detector technology, capable of high spatial resolution volumetric imaging.11) Using this ACT imaging may enable adequate visualization of stent position and especially the stent struts.2)3)
Even though this new method needs to be evaluated in a larger number of patients, it introduces many advantages for patients and surgerons as a new option in the imaging of endovascular treatment and cerebrovacular disease.
References
1. Benitez RP, Silva MT, Klem J, Veznedaroglu E, Rosenwasser RH. Endovascular occlusion of wide-necked aneurysms with a new intracranial microstent (Neuroform) and detachable coils. Neurosurgery. 2004; 6. 54(6):1359–1367. discussion 1368. PMID: 15157292.

2. Benndorf G, Claus B, Strother CM, Chang L, Klucznik RP. Increased cell opening and prolapse of struts of a neuroform stent in curved vasculature: value of angiographic computed tomography: technical case report. Neurosurgery. 2006; 4. 58(4 Suppl 2):ONS-E380. discussion ONS-E380.
3. Benndorf G, Strother CM, Claus B, Naeini R, Morsi H, Klucznik R, et al. Angiographic CT in cerebrovascular stenting. AJNR Am J Neuroradiol. 2005; 8. 26(7):1813–1818. PMID: 16091535.
4. Buhk JH, Lingor P, Knauth M. Angiographic CT with intravenous administration of contrast medium is a noninvasive option for follow-up after intracranial stenting. Neuroradiology. 2008; 4. 50(4):349–354. PMID: 18246336.

5. Grzyska U, Freitag J, Zeumer H. Selective cerebral intraarterial DSA. Complication rate and control of risk factors. Neuroradiology. 1990; 32(4):296–299. PMID: 2234388.
6. Gupta R, Grasruck M, Suess C, Bartling SH, Schmidt B, Stierstorfer K, et al. Ultra-high resolution flat-panel volume CT: fundamental principles, design architecture, and system characterization. Eur Radiol. 2006; 6. 16(6):1191–1205. PMID: 16528556.

7. Howington JU, Hanel RA, Harrigan MR, Levy EI, Guterman LR, Hopkins LN. The Neuroform stent, the first microcatheter-delivered stent for use in the intracranial circulation. Neurosurgery. 2004; 1. 54(1):2–5. PMID: 14683535.

8. Jiang WJ, Xu XT, Du B, Dong KH, Jin M, Wang QH, et al. Long-term outcome of elective stenting for symptomatic intracranial vertebrobasilar stenosis. Neurology. 2007; 3. 68(11):856–858. PMID: 17353474.

9. Lee YJ, Kim DJ, Suh SH, Lee SK, Kim J, Kim DI. Stent-assisted coil embolization of intracranial wide-necked aneurysms. Neuroradiology. 2005; 9. 47(9):680–689. PMID: 16028036.

10. Schueler BA, Kallmes DF, Cloft HJ. 3D cerebral angiography: radiation dose comparison with digital subtraction angiography. AJNR Am J Neuroradiol. 2005; 9. 26(8):1898–1901. PMID: 16155131.
11. SSYLVIA Study Investigators. Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): study results. Stroke. 2004; 6. 35(6):1388–1392. PMID: 15105508.
12. Trossbach M, Hartmann M, Braun C, Sartor K, Hähnel S. Small vessel stents for intracranial angioplasty: in vitro evaluation of in-stent stenoses using CT angiography. Neuroradiology. 2004; 6. 46(6):459–463. PMID: 15127168.

13. Wanke I, Doerfler A, Goericke S, Gizewski ER, Sandalcioglu E, Moemken S, et al. Treatment of wide-necked intracranial aneurysms with a self-expanding stent: mid-term results. Zentralbl Neurochir. 2005; 11. 66(4):163–169. PMID: 16317598.

14. Wanke I, Doerfler A, Schoch B, Stolke D, Forsting M. Treatment of wide-necked intracranial aneurysms with a self-expanding stent system: initial clinical experience. AJNR Am J Neuroradiol. 2003; Jun-Jul. 24(6):1192–1199. PMID: 12812954.
15. Willinsky RA, Taylor SM, TerBrugge K, Farb RI, Tomlinson G, Montanera W. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003; 5. 227(2):522–528. PMID: 12637677.

Fig. 1
(A) Stenosis, 85% (B) Precise 8 × 40 mm stent insertion. (C) Preop IA-ACT shows calcified plaque (arrow). (D) On axial views on post op IA and IV-ACT, the level of the plaque are showen. They further reveal the circumferential narrowing by calcified plaque (arrow). (E) Lateral ACT view (1 mm sections) provide superior visualization of the entire stent its strus and the remanining narrowing and show the underlying, heavialy calcified plaque(arrowhead) responsible for incomplete stent deployment. IA = intrarterial; ACT = angiographic computed tomography; IV = intravenous.
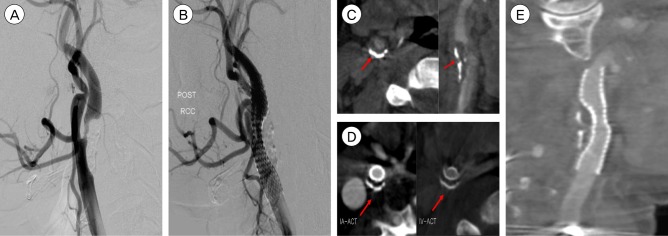
Fig. 2
(A) Severe stenosis of the right M1 segment. (B) Treatment with 2.5 × 8 mm durg eluting stent (Endeavor; Medtronic scientific, Roncadelle (BS), Italy) with successful, completely reestablishing the lumen (C) IA-ACT immediately after procedure show clear visualization of the stent and distal patency, also show no acute thrombosis. (D) IV-ACT after 3 month later show no instent stenois and clear stent struct. IA = intrarterial; ACT = angiographic computed tomography; IV = intravenous.
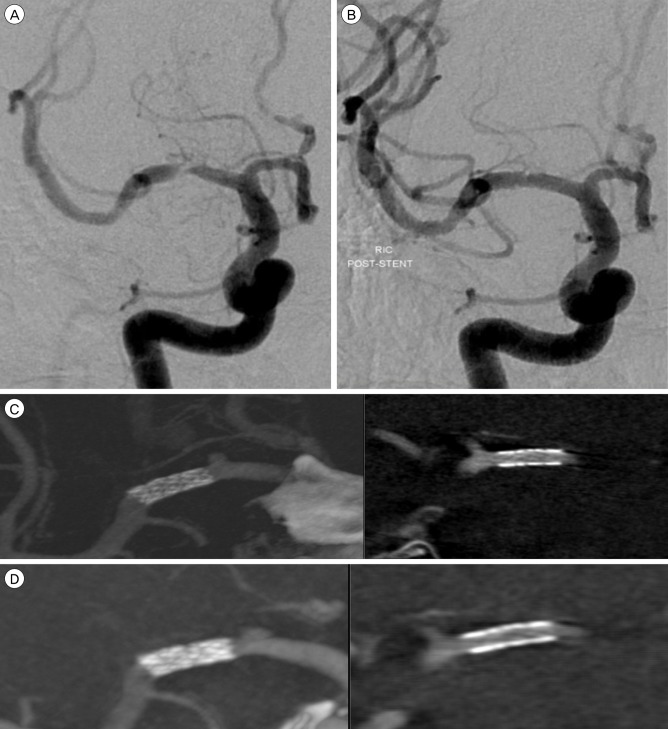
Fig. 3
(A) 3D DSA show unruptured aneurysm on paraclinoid aneurysm. (B) IA-ACT is performed after stent deployment and before coil embolizatoin. Excellent stent distal and proximal marker visibility and good position of stent is shown. (C) ACT image is performed after coil embolization allows definite exclusion of stent strut movement or coil protrusion with minimal beam-hardening artifacts. DSA = digital subtraction angiography; ACT = angiographic computed tomography.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download