Abstract
Objective
The gyrus rectus (GR) is known as a non-functional gyrus; hence, its resection is agreed to be a safe procedure frequently practiced to achieve a better surgical view during specific surgeries. This study aimed at comparing the cognitive outcomes following GR resection in patients who underwent surgery for ruptured anterior communicating artery (ACoA) aneurysms.
Materials and Methods
From 2012 to 2015, 39 patients underwent surgical clipping for ruptured ACoA aneurysms. Mini-mental state examinations (MMSE) were performed in 2 different periods. The statistical relationship between GR resection and MMSE results was evaluated, and further analysis of MMSE subgroup was performed.
Results
Twenty-five out of the 39 patients (64.19%) underwent GR resection. Mean initial and final MMSE scores in the GR resection group were 16.3 ± 9.8 and 20.8 ± 7.3, respectively. In the non-resection group, the mean initial and final MMSE scores were 17.1 ± 8.6 and 21.9 ± 4.5, respectively. Neither group's scores showed a significant change. Subgroup analysis of initial MMSE showed a significant difference in memory recall and language (p = 0.02) but not in the final MMSE scores.
Conclusion
There was no significant relationship between the GR resection and cognitive outcomes in terms of total MMSE scores after surgery for ruptured ACoA aneurysm. However, subgroup analysis revealed a temporary negative effect of GR resection in the categories of language and memory recall. This study suggests that GR resection should be executed superficially, owing to its close anatomical relationship with the limbic system.
Patients who recover from the treatment of ruptured aneurysms in the frontal basal region, without clinical signs of neurological impairment, may show evidence of cognitive dysfunction upon neuropsychological tests.10)18) It has been reported that surgeries in this region, e.g., in cases of ruptured anterior communicating artery (ACoA) aneurysms, can lead to post-operative deficits including impairment in memory, language function, executive function, and change in personality.16) Although considerable importance is attributed to the initial insult caused to the brain by the aneurysm rupture itself and/or consequent vasospasm, other factors, iatrogenic in particular, are expected to affect cognitive outcomes in these patients.
The gyrus rectus (GR) is a non-functional and primitive gyrus; hence, resection of this gyrus is agreed to be a safe surgical procedure, frequently practiced to achieve a better surgical view of the frontal basal region. This study questioned the safety of this procedure concerning its anatomical proximity of the GR to the limbic system, and collected and analyzed data of the patients who underwent this procedure. Furthermore, since memory and language functions are regulated in the dominant frontotemporal lobes, and surgical approach to the ACoA aneurysm involves dissection and manipulation of the frontotemporal lobes, we hypothesized that the direction of surgical approach may have an effect on the cognitive functions of these patients. The main purpose of this study is to compare the cognitive outcomes following GR resection using mini-mental state examinations (MMSE) in patients who underwent clipping surgery for ruptured ACoA aneurysms.
Patients with aneurysmal subarachnoid hemorrhage (SAH) treated between 2012 and 2015 at our institutions were enrolled in the study. A review of retrospective data of these patients was performed and patients were further selected according to the following inclusion criteria: 1) aneurysm location at the ACoA origin, 2) Computed Tomography (CT) angiography with or without Digital Subtraction Angiography (DSA) showing no vascular malformation or other non-aneurysmal sources of hemorrhage, 3) early surgery by aneurysm neck clipping, 4) not older than 65 years of age, 5) clinical state according to Hunt and Hess scale I-IV on admission, 6) clinical state eligible for MMSE at least once during the admission period, and 7) absence of known prior psychiatric history.
Among the initial pool of 265 patients with aneurysmal SAH, 131 underwent surgical clipping, while 134 underwent endovascular treatment and; hence, they were excluded. Among the surgically treated patients, 43 had aneurysms of an ACoA origin. Two patients were excluded since they were at an advanced age, and 2 more were excluded because they were in a grave state and MMSE could not be undertaken. In total, 39 patients were selected as final study subjects. Their hospital data on age, sex, initial radiologic and clinical state, operative findings, clinical course, and cognitive functions were reviewed.
The initial neurological status and severity of SAH were assessed using the Hunt and Hess scale and the Modified Fisher scale, respectively.8)12) Patients were not randomized according to the direction of craniotomy; however, right-sided approaches were used when feasible. A left-sided approach was used when the aneurysmal neck and the feeding arteries were more easily accessible from the left. Partial resection of the GR was performed in patients with high positioned aneurysms or in patients with severe brain swelling and limited surgical view.
The patients were divided into 2 groups based on the type of GR resection during the surgery. Their serial MMSE results were recorded, and the 2 groups were compared and statistically analyzed to validate their significance. Another set of analyses was undertaken to investigate whether the direction of the surgical approach had an effect on the MMSE results of the 2 groups. Further, subgroup analysis was performed for specific categories of MMSE, namely orientation, memory registration, calculation, memory recall, and language. Patients were also grouped according to the direction of the surgical approach, and an association analysis was performed in order to investigate the relationship between the direction of surgery and MMSE results. Total number of patients with symptomatic vasospasm was noted, and the effect of vasospasm on the patients' MMSE results was also investigated.
The statistical analysis was generated using the IBM SPSS Statistics Version 22 (IBM Corp., Armonk, NY, USA). The data were analyzed with the Chi-square test, Fisher's exact test, t-test and the Mann-Whitney test. For the association analysis, bivariate correlation tests were used. For all analyses, p < 0.05 was deemed significant.
The patients were 16 women and 23 men (ratio 1:1.44), and the mean age was 52.2 ± 8.8 years (range, 29-65 years). Twenty-five of 39 patients (64.19%) underwent GR resection. Their demographics and clinical characteristics are compared in Table 1. The patients' demographics, initial neurological status, and SAH severity at the initial CT scan showed no significant difference between the 2 groups.
The initial MMSE was assessed on a mean of 15.2 days after the surgery while the final MMSE was assessed on a mean of 44.2 days after the surgery. The mean initial and final MMSE scores were 16.6 ± 9.4 and 21.2 ± 7.3, respectively. In the GR resection group, the mean initial and final MMSE scores were 16.3 ± 9.8 and 20.8 ± 7.3, respectively. In the non-resection group, the mean initial and final MMSE scores were 17.1 ± 8.6 and 21.9 ± 4.5, respectively. Although the values were slightly higher in the non-resection group, there was no significant difference (p = 0.79 and p = 0.65, respectively).
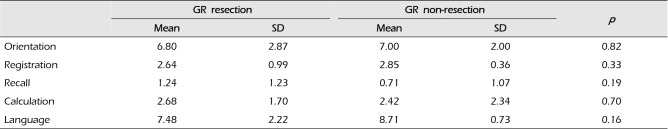
Subgroup analyses concerning each category that constitutes the MMSE, namely orientation, memory registration, memory recall, calculation, and language were performed and the results are shown in Tables 2 and 3. In the initial MMSE, scores for all 5 categories were lower in the GR resection group than in the non-resection group. Among these, only the differences in the memory recall (0.29 vs. 0.96; p = 0.02) and language (9.96 vs. 7.14; p = 0.02) categories were significant. However, in the final MMSE, none of the sub-categories showed a significant difference.
Nineteen patients underwent surgery from the right side and 20 from the left. In the association analysis between the direction of surgical approach and MMSE, right-sided craniotomy showed a negative, but not significant (β = -7.86, SE = 4.12, p = 0.08), relationship with the MMSE.
We also analyzed the relationship between the vasospasm and patient cognitive outcome after surgery using MMSE. Seven of 39 patients showed vasospasm after surgery. In the vasospasm group, initial and final mean MMSE scores were 16.7 ± 10.4 and 21.8 ± 5.2, respectively. In the non-vasospasm group, mean initial and final MMSE scores were 16.5 ± 9.4 and 21.0 ± 6.8, respectively. No significant difference was observed between patients in both groups (p = 0.97 and p = 0.77, respectively).
Post-surgical cognitive impairment in ACoA aneurysms may be caused by multiple factors that involve the orbital prefrontal cortex lesions or a disconnection in the ventromedial circuits.1)5)6)13) The close proximity of these structures to the paraolfactory area, the nucleus accumbens septi and the diagonal band of Broca, makes this zone delicate during ACoA dissection procedures. Ramos et al. reported that in their study, the nucleus accumbens septi was damaged if the dissection was performed over 5 mm in depth from the basal surface.16)
Another anatomical risk may be an injury to the small perforating vessels, originating from the ACoA, that supply the frontal basal region.17) It has been suggested that in patients with ACoA aneurysmal rupture, the cerebral areas traditionally associated with memory functioning are spared, and damage to the basal forebrain and frontal lobes is responsible for memory impairment.3)4)15)
There are other several potential causes of cerebral damage after surgery for ACoA aneurysmal rupture. These include generalized vasospasm with ischemia or infarction, cerebral edema and subsequent mesial temporal lobe herniation, hydrocephalus, and hemorrhagic infarction of the frontotemporal lobes following retraction.11)18) Since language, intellectual, and cognitive functions are controlled in the dominant hemisphere, which is the left hemisphere in most cases, we presumed that the direction of surgical dissection and GR resection may have an effect on patients' cognitive outcomes. However, our results did not show a significant relationship between the direction of surgical approach and the MMSE results. This finding correlates with that of a previous report by Böttger et al., where the side of the lesion did not influence the severity of post-surgical neuropsychological dysfunctions.1)14) They commented that the lack of lateralization, in the case of frontal basal regions, might be explained by the projection of the main output fibers from the fornix to both ipsilateral and contralateral hippocampus.1) Stenhouse et al. reported that the most frequent mechanism of cerebral damage in their patients that underwent surgery for ACoA aneurysms, was ischemia that presumably resulted from vasospasm.18) We checked the vasospasm using a transcranial doppler daily and additionally evaluated the patients' clinical symptoms. If the vasospasm was confirmed, we conducted angioplasty, using nimodipine. In our study, the relationship between vasospasm and cognitive outcome was not significant. Moreover, the study sample was too small to comment on the effects of such potential mechanisms with certainty.
The sub-categories of memory recall and language being lower in the GR resection group in the initial, but not the final, MMSE may be explained by short-term neuroplasticity of the brain. Many studies have reported substantial changes in frontal white matter and increased cognitive function after exposure to repeated training or experience, in brains of patients with pathologic conditions and in elderly patients with age-related changes.7)19) Moreover, Takeuchi et al. reported in their recent studies the positive impact of working-memory training on the frontoparietal gray matter.20) Similarly, repeated exposure to auditory, verbal, and visual stimulation and repeated neurocognitive examinations during admission may have accelerated neuroplasticity and resulted in such a change in MMSE results.
There are several limitations in this study. First, this was a retrospective study; therefore, the patients were not randomized. There may have been a selection bias and GR may have been resected in cases of severe brain swelling, suggesting poorer baseline cognitive functions in these patients. Second, a small sample size limited the significance of the results. Further larger sampled, anterograde, and randomized studies are needed to prove a stronger correlation between GR resection and postsurgical cognitive outcomes. Third, MMSE may be a simple and handy questionnaire that can be done anywhere in a short period; however, it might be insufficient to interpret a patient's detailed cognitive status. Moreover, a learning effect after repeated tests may have masked the patients' true cognitive status.2)9) A more focused and complex neuropsychiatric test may need to be applied in future studies to overcome such limitations.
In general, there was no significant correlation between the GR resection and cognitive outcomes in terms of total MMSE scores after surgery for ruptured ACoA aneurysm. However, subgroup analysis showed a negative effect of GR resection on categories of language and memory recall. Although limited by a small sample size, this study suggests contemplating to perform the GR resection superficially, considering its close anatomical proximity to the limbic system.
References
1. Böttger S, Prosiegel M, Steiger HJ, Yassouridis A. Neurobehavioural disturbances, rehabilitation outcome, and lesion site in patients after rupture and repair of anterior communicating artery aneurysm. J Neurol Neurosurg Psychiatry. 1998; 7. 65(1):93–102. PMID: 9667568.
2. Claus JJ, Mohr E, Chase TN. Clinical trials in dementia: learning effects with repeated testing. J Psychiatry Neurosci. 1991; 3. 16(1):1–4. PMID: 2049365.
3. Damasio AR, Graff-Radford NR, Eslinger PJ, Damasio H, Kassell N. Amnesia following basal forebrain lesions. Arch Neurol. 1985; 3. 42(3):263–271. PMID: 3977657.

4. DeLuca J. Cognitive dysfunction after aneurysm of the anterior communicating artery. J Clin Exp Neuropsychol. 1992; 11. 14(6):924–934. PMID: 1452638.

5. Diamond BJ, DeLuca J, Kelley SM. Memory and executive functions in amnesic and non-amnesic patients with aneurysms of the anterior communicating artery. Brain. 1997; 6. 120(Pt 6):1015–1025. PMID: 9217685.

6. Ebeling U, von Cramon D. Topography of the uncinate fascicle and adjacent temporal fiber tracts. Acta Neurochir (Wien). 1992; 115(3-4):143–148. PMID: 1605083.

7. Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth Ø, Larsen VA, et al. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum Brain Mapp. 2012; 10. 33(10):2390–2406. PMID: 21823209.

8. Frontera JA, Claassen J, Schmidt JM, Wartenberg KE, Temes R, Connolly ES Jr, et al. Prediction of symptomatic vasospasm after subarachnoid hemorrhage: the modified fisher scale. Neurosurgery. 2006; 7. 59(1):21–27. discussion 21-7. PMID: 16823296.
9. Galasko D, Abramson I, Corey-Bloom J, Thal LJ. Repeated exposure to the Mini-Mental State Examination and the Information-Memory-Concentration Test results in a practice effect in Alzheimer's disease. Neurology. 1993; 8. 43(8):1559–1563. PMID: 8351011.

10. Hillis AE, Anderson N, Sampath P, Rigamonti D. Cognitive impairments after surgical repair of ruptured and unruptured aneurysms. J Neurol Neurosurg Psychiatry. 2000; 11. 69(5):608–615. PMID: 11032612.

11. Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968; 1. 28(1):14–20. PMID: 5635959.

12. Hütter BO, Gilsbach JM. Cognitive deficits after rupture and early repair of anterior communicating artery aneurysms. Acta Neurochir (Wien). 1992; 116(1):6–13. PMID: 1615771.
13. Krisht AF. Anterior communicating artery aneurysms: techniques in surgical clipping. Neurosurgery. 2001; 7. 23(15):1–5.
14. Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993; 3. 362(6418):342–345. PMID: 8455719.

15. Phillips S, Sangalang V, Sterns G. Basal forebrain infarction. A clinicopathologic correlation. Arch Neurol. 1987; 11. 44(11):1134–1138. PMID: 3675245.
16. Ramos A, Chaddad-Neto F, Joaquim AF, Campos-Filho JM, Mattos JP, Ribas GC, et al. The microsurgical anatomy of the gyrus rectus area and its neurosurgical implications. Arq Neuropsiquiatr. 2009; 3. 67(1):90–95. PMID: 19330219.

17. Ravnik J, Starovasnik B, Sesok S, Pirtosek Z, Svigelj V, Bunc G, et al. Long-term cognitive deficits in patients with good outcomes after aneurysmal subarachnoid hemorrhage from anterior communicating artery. Croat Med J. 2006; 4. 47(2):253–263. PMID: 16625690.
18. Stenhouse LM, Knight RG, Longmore BE, Bishara SN. Long-term cognitive deficits in patients after surgery on aneurysms of the anterior communicating artery. J Neurol Neurosurg Psychiatry. 1991; 10. 54(10):909–914. PMID: 1744646.

19. Takeuchi H, Sekiguchi A, Taki Y, Yokoyama S, Yomogida Y, Komuro N, et al. Training of working memory impacts structural connectivity. J Neurosci. 2010; 3. 30(9):3297–3303. PMID: 20203189.

20. Takeuchi H, Taki Y, Sassa Y, Hashizume H, Sekiguchi A, Fukushima A, et al. Working memory training using mental calculation impacts regional gray matter of the frontal and parietal regions. Plos One. 2011; 8. 6(8):e23175. PMID: 21886781.

Table 1
Clinical characteristics of the patients
Table 2
Comparison of initial MMSE sub-categories between gyrus rectus resection and non-resection groups
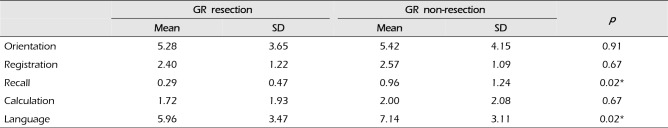
Table 3
Comparison of final MMSE sub-categories between gyrus rectus resection and non-resection groups




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download