Abstract
Objective
To report effects of the pre-procedural rehydration for reduce thromboembolic complications in acute phase aneurysmal subarachnoid hemorrhage coil embolization.
Materials and Methods
From January 2009 to December 2013, 190 patients with ruptured aneurysmal subarachnoid hemorrhage (aSAH) treated by coil embolization at our institution were consecutively enrolled in this study. In period 1 (from January 2009 to June 2012, n = 122), pre-procedural fluid was not supplied. In period 2 (from July 2012 to December 2013, n = 68), depending on the state of the patient's body weight and degree of dehydration, intravenous fluid was started with infusion of approximately 7 mL/kg of 0.9 percent saline (minimum 300 to maximum 500 mL) over 30 minutes.
Results
A total of 190 patients were hospitalized due to aSAH and underwent coil embolization for five years between January 2009 and December 2013. Of these, 122 patients underwent coil embolization based on the old protocol before June 2012 (period 1) and 68 underwent the procedure based on the new protocol after the period 2. Neck size, width, maximum diameter of the aneurysm and procedure time were associated with procedure related thromboembolic complications in entire periods (multivariate analysis, p < 0.05, in respectively). The frequency of thromboembolism showed a drastic decrease in period 2 (re-hydration period), from 18.0% (22/123) to 4.4% (3/67), which was also statistically significant (p = 0.007, Chi-square test).
Endovascular coil embolization is one of the techniques used for treatment of cerebral aneurysm. Platinum coils are inserted into the lumen of the aneurysm, so that a local thrombus is formed around the coils, obliterating the aneurysmal sac.4) Coil embolization, which is performed through the intravascular space, has an inherent risk of causing thromboembolism. Thromboembolic complications occur more frequently in ruptured aneurysm that cannot be treated with antiplatelet medication and intra-procedural administration of heparin compared to unruptured cases.8)10) Such pre-medications (antiplatelet and anticoagulant administration) can reduce the occurrence of thromboembolism in treatment of unruptured aneurysm.5) For ruptured aneurysmal subarachnoid hemorrhage (aSAH), coil embolization is mainly performed in unplanned emergency. However, pre-medication of antiplatelet or heparin prior to emergency coil embolization in ruptured aSAH is under many restrictions in real clinical practice, because it is possible to increase the risk of rebleeding. Therefore, coil embolization for ruptured aneurysm may inevitably cause more thromboembolic complications than unruptured aneurysm. Also, it is widely assumed that dehydration predispose to thromboembolism after acute stroke.6) Emergency coil embolization in dehydration state, it may be more dangerous. We assumed that calibrated the dehydration be able to reduce the risk of thromboembolic complication in acute phase coil embolization. Contrary to the therapeutic treatment, administering a proper dose of fluid to the patient is very easy and simple. It may correct dehydration, causing no additional complication for the patient, and is expected to reduce thromboembolic complications. We examined patients who were to under emergency coil embolization for aSAH, assuming that administering a proper dose of fluid to the patients would reduce the risk of thromboembolism without additional complications.
From January 2009 to December 2013, 190 patients with ruptured aSAH treated by coil embolization at our institution were consecutively enrolled in this study. Subarachnoid hemorrhage was confirmed by computed tomography, magnetic resonance image, or cerebrospinal fluid analysis. Coil embolization procedures were performed under monitored anesthesia by three experienced operators. At least two of the three experienced physicians were involved in each procedure. Basically there was no significant difference between operators. In general, neither antiplatelet premedication nor systemic heparinization was administered during the procedure, until proper protection of the ruptured aneurysm was achieved (no contrast filling within the aneurysmal dome). After confirming of proper protection of ruptured aneurysm (no contrast filling in the top of the aneurysm), a bolus of 3000 IU of heparin was administered intravenously. And additional 1000 IU bolus of heparin was administered every hour.
Thromboembolic complication was defined as any event with complete or partial occlusion of arteries, detection of low density or high signal on postoperative CT or MR consistent with patient's symptoms.
In period 1 (from January 2009 to June 2012, n = 122), pre-procedural fluid was not supplied. In period 2 (from July 2012 to December 2013, n = 68), depending on the state of the patient's body weight, intravenous fluid was started with infusion of approximately 7 mL/kg of 0.9 percent saline (minimum 300 to maximum 500 mL) over 30 minutes.
No variation in the treatment protocol was observed between the two periods and the coil embolization was performed by three experienced operators. At least two of these three physicians were engaged together in performance of each procedure.
Statistical analysis was performed using the SPSS statistical package (SPSS for Windows, version 20.0, Chicago, IL, USA). The Chi-square, Student t-test and univariate logistic regression were used for comparisons as appropriate. Variables with p value < 0.05 in univariate analysis were selected for the multivariable model using multiple logistic regression analysis.
A total of 190 patients were hospitalized due to aSAH and underwent coil embolization for five years between January 2009 and December 2013. Of these, 122 patients underwent coil embolization based on the old protocol (Period 1, January 2009 to June 2012) and 68 underwent the procedure based on the new one after the period (period 2, July 2009 to December 2013). No difference in the patient's age, gender, past medical history, Glasgow coma scale, World Federation of Neurosurgical Societies grading, size of aneurysm, or procedure time for coil embolization was found between the two periods. (Table 1)
In Period 2, coil embolization was performed approximately six hours (368.99 ± 243.54 minutes) after the occurrence of symptoms and 417 ± 93.21 mL fluid was administered pre-procedurally. The effectiveness of the coil embolization procedure using a single microcatheter was greater with statistical significance in Period 1 (87%; 107/122) than in Period 2 (69.1%, 47%) (p = 0.003). No statistically significant difference in the rate of intra-procedural rupture considered to be a complication of coil embolization was found between Period 1 (11.5%; 14/122) and Period 2 (14.7%; 10/68) (p = 0.506). With an additional use of microcathter, thromboembolic complications could be increased. In this study, there are more frequent use of additional microcatheter or balloon and stent in period 2. However, the frequency of thromboembolism showed a drastic decrease, from 18.0% (22/123) before the protocol to 4.4% (3/67) after the protocol, which was also statistically significant (p = 0.007, Chi-square test). A cardio-pulmonary problem considered to be a complication of pre-procedural fluid administration was found in 14 cases (14/68, 20.6%). Of these, 13 cases improved without sequela and the remaining case required treatment using a ventilator; however, the ventilator care was required due to pulmonary edema before fluid administration (Table 1).
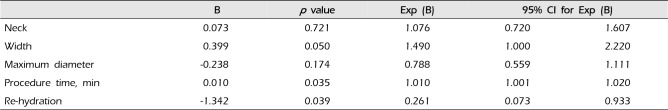
Analysis of thromboembolism occurrence found that the neck size, width, and longest length of aneurysm, pre-procedural fluid supply, and procedure time were statistically significant related factors (univariate logistic regression analysis) (Table 2). In multivariate analysis, the longest length of aneurysm, fluid supply, and length of procedure time were significant related factors (prolonged procedure time (odds ratio [OR] 1.010, 95% confidence interval [CI] 1.001-1.020, p = 0.035) and pre-embolization rehydration (OR 0.261, 95% CI 0.073-0.993, p = 0.039) were independent risk factors for thromboembolic complication in multivariate logistic regression analysis) (Table 3). Pre-embolization rehydration, turned out to be a factor that could be reduce the procedural thromboembolic complications.
Aneurysmal subarachnoid hemorrhage is a potentially fatal disease with high morbidity and mortality. Only a third of aSAH patients may improve and many such patients can die or become disabled.1)2) In ruptured aneurysm, if left untreated, the risk of rebleeding within the first 24 hours is 3-4%1); since rebleeding is the most important factor preventing the patient's recovery, it is widely accepted that a ruptured aneurysm requires emergency treatment.
Since the International Subarachnoid Aneurysm Trial research found that coil embolization was not less effective in treatment of ruptured aneurysm than clipping,7) it has often been used in treatment of ruptured aneurysm and many researchers have reported positive results from the procedure. However, coil embolization is inherently associated with two serious problems: 1) intra-procedural rupture and 2) intra-procedural thromboembolism. It can involve these two complications more frequently compared with the unruptured aneurysm.3)8)11)12) Prospective large-scale research found that coil embolization for aSAH caused rupture in 3.2% to 7.6% of cases and thromboembolism in 11% to 13.3%.3)8) The frequency of thromboembolism is approximately twice as high as that for unruptured aneurysm (6.7-7.3%).8)10) Larger aneurysm, a wider aneurismal neck, or use of diverse instruments may lead to higher risk of thromboembolism.9)10) Pre-procedural ample supply of fluid and antiplatelet preparation, intra-procedural heparin administration, intra-procedural thrombolysis when thrombus occurs, and additional post-procedural anticoagulant or antiplatelet medication are used to reduce the problems in case of coil embolization for unruptured cerebral aneurysm and such treatments are known to reduce the frequency of thromboembolism.5) Patients with unruptured aneurysm are administered a good dose of fluid to prevent dehydration and two antiplatelet agents for at least five days pre-procedurally, as well as heparin intra-procedurally, thus they may inevitably be at lower risk of thromboembolism than those with aSAH. For ruptured aneurysm, which is treated with unplanned emergency coil embolization, administration of sufficient pretreatment is difficult and the likelihood of rebleeding may place restrictions on antithrombotic administration, consequently causing more thromboembolic complications. However, administration of antiplatelet or heparin in clinical practice with the aim of reducing the high risk of thromboembolism for ruptured aneurysm is difficult. In cases of unruptured aneurysm, more aggressive strategies could be tried for prevention or removal of thrombus. However, coil embolization in ruptured cases has significant limitation compared to unruptured cases, due to the emergency situation for adequate preparation or the high risk of rebleeding of aneurysm or predilection for bleeding due to the fragile nature of the brain caused by antecedent intracerebral hemorrhage.
While some aSAH patients may visit emergency rooms in tertiary hospitals immediately after occurrence of symptoms, many of them may be transferred to a tertiary hospital from primary and secondary hospitals to undergo coil embolization after aneurysm rupture. For this reason, performance of coil embolization after the condition occurs may take much more time. Because most patients are under nothing by mouth during transfer, they are likely to be dehydrated unless provided with an adequate dose of fluid. Enhanced computed tomography and digital subtraction angiography using contrast during can aggravate dehydration. Performing invasive angiography and coil embolization in those patients without supply of fluid is more likely to cause thromboembolism. In this study it took six hours on average for the patients to be transferred to a hospital and undergo coil embolization; during this period of time, most of them were prevented from drinking any fluid and did not have a proper intravenous supply of fluid. We found that simply providing patients with a proper dose of fluid pre-procedurally could reduce thromboembolism.
Because many other factors can cause thromboembolism, it may be difficult to say that this method is sufficient to reduce the frequency of thromboembolism. In addition to the factors of aneurysm itself (size of aneurysm, size of its neck, etc.), as mentioned above, using additional microcatheter or inserting a stent is known to increase the frequency of such complications. We also found that the neck size, width, and longest length of the aneurysm and procedure time were associated with the occurrence of thromboembolism. In this study, however, the frequency of thromboembolism was lower in Period 2, although an additional microcatheter was used more frequently than in Period 1. This result could be suggest that becoming more experienced may lead to technical improvement so that use of additional instruments, such as additional microcatheter and stents, does not increase the frequency of thromboembolism; however, no variation in the frequency of intra-procedural rupture between the two periods demonstrates that an operator's skill may be dependent neither on prolonged time nor on experience. In addition, the factors based on the skill of individual operators implementing the procedure cannot be neglected. However, since at least two of the three physicians in this study were engaged together in performance of each procedure of coil embolization, it is believed that variation based on the differences among the individual doctors can be excluded. Therefore it can be said that the variation in the frequency of thromboembolism between the two periods was significantly affected by the pre-procedural supply of fluid.
The most frequent complication of pre-procedural rehydration for ruptured aneurysm was a pulmonary complication. However it was cured in most cases, leaving no sequela. No statistically significant difference was found in the frequency of intra-procedural rupture caused by fluid injection.
The most significant finding from this study is that an ample supply of fluid in coil embolization for ruptured aneurysm is likely to reduce the risk of thromboembolism and is unlikely to cause additional complications.
It goes without saying that this study has many limitations: first, the patients were gathered prospectively in two separate periods but were not assigned randomly and were analyzed retrospectively; second, although similar complications of ruptured aneurysm were found between the two groups, the possibility that the accumulation of an operator' experiences led to lower frequency of thromboembolism cannot be completely excluded. And, quantitative analysis degree of the patients' dehydration is lacking. The authors checked degree of dehydration by subjective patients' felling of thirsty and the dryness of the tongue.
Since administering a sufficient dose of fluid according to the degree of pre-procedural dehydration for the patient is very simple in a methodological way and is unlikely to cause additional complications, it is worth trying. Nevertheless, since this study has many limitations, as mentioned above, care should be taken in interpreting the results and prospective research should be conducted to confirm them.
Pre-procedural administration of a sufficient dose of fluid considering the patient's dehydration reduced the frequency of thromboembolism in cases of emergency coil embolization for ruptured aneurysm, without increasing additional specific complications. While this result has come from retrospective research and care should be taken in interpreting it, the pre-procedural supply of fluid is very easy and simple and can be employed with ease in clinical practice.
References
1. Bederson JB, Connolly ES Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009; 3. 40(3):994–1025. PMID: 19164800.

2. Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994; 7. 25(7):1342–1347. PMID: 8023347.

3. Cognard C, Pierot L, Anxionnat R, Ricolfi F. Clarity Study Group. Results of embolization used as the first treatment choice in a consecutive nonselected population of ruptured aneurysms: clinical results of the Clarity GDC study. Neurosurgery. 2011; 10. 69(4):837–841. discussion 842. PMID: 21623247.
4. Guglielmi G, Vinuela F, Sepetka I, Macellari V. Electrothrombosis of saccular aneurysms via endovascular approach. Part 1: Electrochemical basis, technique, and experimental results. J Neurosurg. 1991; 7. 75(1):1–7. PMID: 2045891.
5. Kang HS, Han MH, Kwon BJ, Jung C, Kim JE, Kwon OK, et al. Is clopidogrel premedication useful to reduce thromboembolic events during coil embolization for unruptured intracranial aneurysms? Neurosurgery. 2010; 11. 67(5):1371–1376. discussion 1376. PMID: 20871459.

6. Kelly J, Hunt BJ, Lewis RR, Swaminathan R, Moody A, Seed PT, et al. Dehydration and venous thromboembolism after acute stroke. QJM. 2004; 5. 97(5):293–296. PMID: 15100423.

7. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, et al. International Subarachnoid Aneurysm Trial (ISAT) Collaborative Group. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. J Stroke Cerebrovasc Dis. 2002; Nov-Dec. 11(6):304–314. PMID: 17903891.

8. Park HK, Horowitz M, Jungreis C, Genevro J, Koebbe C, Levy E, et al. Periprocedural morbidity and mortality associated with endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol. 2005; 3. 26(3):506–514. PMID: 15760857.
9. Pierot L, Cognard C, Anxionnat R, Ricolfi F. CLARITY Investigators. Endovascular treatment of ruptured intracranial aneurysms: factors affecting midterm quality anatomic results: analysis in a prospective, multicenter series of patients (CLARITY). AJNR Am J Neuroradiol. 2012; 9. 33(8):1475–1480. PMID: 22517279.

10. Pierot L, Spelle L, Vitry F. ATENA: the first prospective, multicentric evaluation of the endovascular treatment of unruptured intracranial aneurysms. J Neuroradiol. 2008; 5. 35(2):67–70. PMID: 18466978.

11. Pierot L, Spelle L, Vitry F. ATENA Investigators. Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke. 2008; 9. 39(9):2497–2504. PMID: 18617659.
12. Pierot L, Wakhloo AK. Endovascular treatment of intracranial aneurysms: current status. Stroke. 2013; 7. 44(7):2046–2054. PMID: 23798560.
Table 1
Demography of patients during the two periods
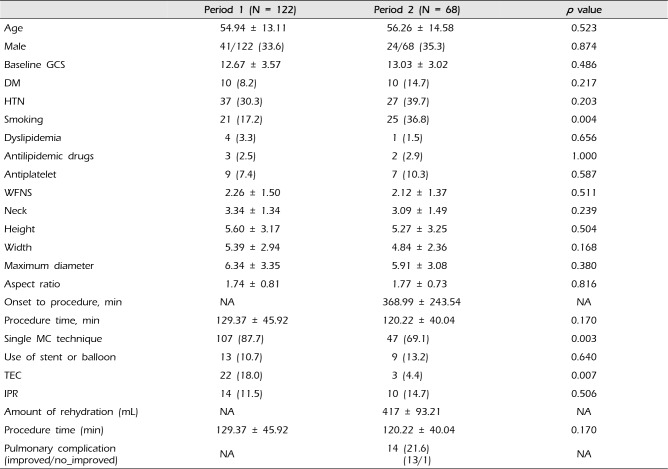
Table 2
Risk factors of thromboembolic complication by univariate logistic regression
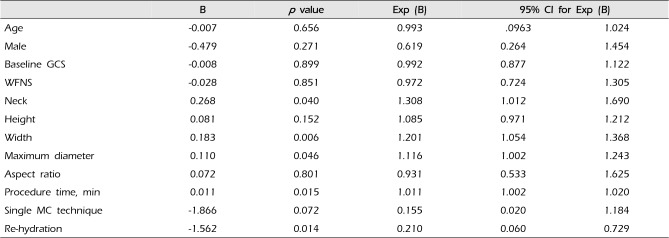
Table 3
Risk factors of thromboembolic complication by multivariate logistic regression




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download