Abstract
For securing large, giant, and wide-neck aneurysms, conventional coil embolization has substantial limitations, such as incomplete occlusion, recanalization, and a high recurrence rate. To overcome these limitations, a novel paradigm was suggested and, as a result, flow-diverting device was developed. The flow-diverting device is an innovative and effective technique to allow securing of large, giant, and wide-neck aneurysms. In numerous studies, the flow-diverting device has shown better outcomes than coil embolization. However, the flow-diverting device has also some risks, including rupture of aneurysm, intracerebral hemorrhage, and ischemic stroke. In addition, with more experience, unexpected complications are also reported.5)7) In the present case, we experienced a delayed ischemic stroke at 27 days after endovascular treatment. The patient had multiple aneurysms and, among them, we treated a large posterior communicating artery aneurysm using Pipeline™ Embolization Device. The patient was tolerable for 25 days, but then suddenly presented intermittent right hemiparesis. In the initial diffusion magnetic resonance imaging (MRI), there was no acute lesion; however, in the follow-up MRI, an acute ischemic stroke was found in the territory of anterior choroidal artery which was covered by Pipeline Embolization Device. We suspect that neo-intimal overgrowth or a tiny thrombus have led to this delayed complication. Through our case, we learned that the neurosurgeon should be aware of the possibility of delayed ischemic stroke after flow diversion, as well as, long-term close observation and follow-up angiography are necessary even in the event of no acute complications.
The endovascular treatment of intracranial aneurysms has become an alternative technique of surgical clipping for 25 years.9)18) However, coil embolization still has limitations, especially, in wide-neck, large, and giant aneurysm cases.2)5)9)13) To overcome these limitations, the flow-diverting device was developed and introduced for the treatment of irresoluble aneurysms.10) After the efficacy and safety of the flow-diverting device has been demonstrated in several studies, it has generally been accepted as a new solution for large or wide-neck aneurysms. Although the flow-diverting device has low recanalization rate and low recurrence rate, it also has severe and unexpected complications, such as ischemic stroke, spontaneous rupture of aneurysm, intracerebral hemorrhage, and stent stenosis.1)2)8)11)17)19) Among these complications of the flow-diverting device, ischemic stroke due to thromboembolism and perforator occlusion is well-described and occurs most frequently. In previous reports, ischemic strokes were reported to occur in 2.5-13% of patients and most of the instances occurred during the endovascular treatment.1)3)11)17) Ischemic stroke may result from insufficient antiplatelet therapy, stent wall thrombus formation and occlusion, parent artery occlusion or distal thromboembolic events. It should also be noted that this complication was more frequent in posterior circulation and giant aneurysm cases.1)17)
In the present case, we observed a delayed ischemic stroke in the territory of the anterior choroidal artery which was covered by the flow-diverting device for the treatment of a large posterior communicating artery aneurysm.
A 56 year-old male with histories of cerebral palsy, craniotomy for head trauma, and acute myocardial infarction, presented at our hospital for incidentally detected aneurysms. Because of cerebral palsy, he already had right hemiparesis (motor grade IV, modified Rankin Scale (mRS) score 2), but other neurologic examinations were intact. In his brain MRA, there were four aneurysms on the right and left middle cerebral artery (MCA) bifurcation, anterior communicating artery (ACoA), and left posterior communicating artery (PCoA). The bilateral MCA bifurcation and ACoA aneurysms were smaller than 5 mm, but the left PCoA aneurysm was measured 19 mm.
Due to the history of myocardial infarction, the patient already had administered aspirin and clopidogrel for one year. Prior to the endovascular treatment, we performed a drug resistance test for aspirin and clopigrel. Aspirin resistance test was 387 ARU (Aspirin Reaction Unit, normal range: < 550, aspirin resistance: ≥ 550) and clopidogrel resistance test was 120 PRU (P2Y12 Reaction Unit, normal range: < 240 PRU, clopidogrel resistance: ≥ 240 PRU). These results confirmed that the patient does not had resistance to aspirin and clopidogrel.
We conducted a diagnostic transfemoral cerebral angiography (TFCA) under local anesthesia. In TFCA, the large PCoA aneurysm was located between the left PCoA and the left anterior choroidal artery (AChA), and had a small daughter sac in the dome of the aneurysm. The left AChA was separated from the large aneurysm, but the proximal PCoA originated from the neck of the large aneurysm. Since the large aneurysm was wide-neck and involved the proximal PCoA, we decided to reschedule the operation for deploying the flow-diverting device at the large aneurysm and conventional coiling at the other aneurysms (Fig. 1).
Under general anesthesia, the operation was performed under intravenous heparin infusion with the goal of maintaining an activated clotting time at 2 times of the normal value. After the puncture of the right common femoral artery, 7-French Flexor shuttle® guiding sheath (Cook Medical, Bloomington, IL, USA) was placed in the left internal carotid artery (ICA) and the left ACoA and MCA bifurcation aneurysms were managed first using micro-catheters and coils. After the successful coiling of the left ACoA, MCA bifurcation aneurysms, the Marksman™ Micro Catheter (ev3/Covidien, Irvine, CA, USA) was placed on the left proximal MCA and Pipeline™ Embolization Device (PED; ev3/Covidien, Irvine, CA, USA) was deployed from the proximal MCA to the horizontal segment of the cavernous ICA, with covering the AChA and PCoA (Fig. 2). Throughout the course of deployment, the combination of forward pressure and retraction technique was used to maximally attach PED to the ICA wall. The deployment of PED was successfully performed and, in the post-operative angiography, whole branch vessels were not interrupted by PED including PCoA, AChA (Fig. 3). After embolization of the left side aneurysms, the femoral access site was closed with Perclose ProGlide (Abbott Vascular, Santa Clara, CA, USA) and, two days after the endovascular treatment, the patient was discharged with dual antiplatelet therapy, clopidogrel 75 mg, and aspirin 100mg daily, without neurologic deficit.
Dual antiplatelet therapy was continuously maintained and complications of the flow-diverting device were not observed during 25 days. However, 25 days after the endovascular treatment, the patient suddenly presented intermittent right hemiparesis. Although his symptom was not prominent in neurologic examination and he already had right hemiparesis due to previous cerebral palsy, we readmitted him for checking the diffusion magnetic resonance imaging (MRI). In the initial diffusion MRI which was checked at 25 days after the endovascular treatment, an ischemic stroke was not found, so we recommended TFCA for further evaluation. However, he refused more tests and management, because he had phobia about the additional brain exam. We comprehended his phobia because he already had histories of cerebral palsy and craniotomy for head trauma. Because the initial MRI was fine, we maintained dual antiplatelet therapy without additional medication and closely observed his symptoms in the hospital (Fig. 4). However, as time passed, the patient complained of more frequent and worsening hemiparesis, so we rechecked diffusion MRI and MRA. In the second diffusion MRI which was checked at 27 days after the endovascular treatment, ischemic stroke in the territory of the left AChA was observed (Fig. 5) and his symptom, right hemiparesis, was aggravated to motor grade II and mRS score4. We recommended a follow-up TFCA to confirm the occlusion of AChA, but the patient refused again. We maintained dual antiplatelet therapy without additional medication, and transferred him to the department of rehabilitation for physical training of right hemiparesis. He discharged with the dual antiplatelet medication at 83 days after the endovascular treatment. At the point of discharge, right hemiparesis was improved to motor grade III and mRS score 3.
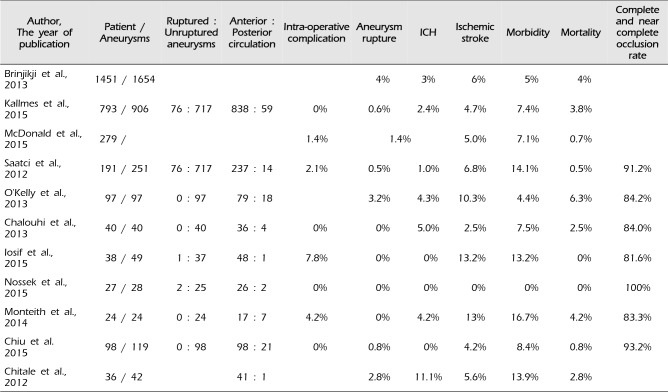
After introducing of the flow-diverting device in 2011, it has become an innovative technique to allow for a more effective and safety endovascular treatment of large, wide-neck, and complex aneurysms. Furthermore, its outcomes also show an excellent occlusion rate, a low recanalization rate, as well as acceptable morbidity and mortality as compared to coil embolization. The flow-diverting device has emerged as an answer to previously irresoluble aneurysms; however, it is not free from some complications. In recent studies, the complications of flow-diverting device, such as branch vessel and/or perforator occlusion, rupture of aneurysm, intracerebral hemorrhage (ICH), stent stenosis and even death were reported (Table 1).1)2)3)4)8)11)13)14)16)17)19)
Numerous single and multicenter studies demonstrated overall rates of adverse complications (Table 1); among these studies, intra-operative and post-operative rupture of aneurysm is a serious concern of flow-diverting device. Although, rupture of aneurysm was reported only 0.6-4%, however, once it occurs, it leads to serious sequelae. In Kallmesstudy,11) the overall rupture rate was only 0.6%; however, the rupture rate was increased with the aneurysm size (giant 4.5%, large 0.6%, small aneurysm 0%). Most of this complication arose in the procedure of wire, micro- catheter manipulation, deployment of device, and balloon inflation for remodeling the device. Therefore, a careful placement of micro-catheter and a gentle manipulation of the device are crucial points for protecting rupture of aneurysm.
Flow diversion-induced ICH is a more specific complication and leads to a significant morbidity and mortality. The incidence of ICH was 1-5% of the patients in several retrospective reports and the exact mechanism is unclear (Table 1). However, dual antiplatelet therapy, hemorrhagic conversion of ischemic stroke, intra-operative hypertension, and altered pressure dynamics are suspected to be causes of ICH.2)17) As all of the above are related to blood pressure, blood pressure should be strictly controlled to prevent hemorrhagic complications.
Ischemic stroke has been reported to be a major complication accounting for 2.5-13.2% of all cases and a vast majority of strokes occurred within 30 days after the endovascular treatment.10) Owing to the thrombogenic nature of the metal component, low porosity of the flow-diverting device, and selective thrombosis in aneurysm sac, ischemic stroke is an expected complication.1)6)13)20) In most studies, the patency of branch vessels and perforators was maintained; however, in overlapping multiple devices, posterior circulation, large and giant aneurysm cases, occurrence risk of ischemic stroke increased more.2)7)11)12)15)16)18) The possible mechanism of ischemic stroke is as follows: intimal hyperplasia with thrombus propagation, insufficient antiplatelet activity, improper deployment, compromise of covered branch, and in-stent stenosis.16)19)
In order to prevent this complication, the dual antiplatelet therapy is required in both preoperative and postoperative settings. Similarly to other endovascular stents, the dual antiplatelet therapy is administered prior to deployment of flow-diverting devices. In most studies,9)14)16)18)19) premedication was performed prior to the operation (aspirin 81-325 mg plus clopidogrel 75 mg for more than five days, or loading aspirin 325-500 mg plus clopidogrel 300-600 mg). After the operation, it is recommended to continue the dual antiplatelet therapy for 3 - 6 months; then, clopidogrel may be stopped depending on follow-up MRA or TFCA and clinical results.4)14)16)19) There are some controversies about the appropriate period of clopidogrel administration. For preventing delayed ischemic strokes and complete occlusion of giant aneurysms, the patients may require clopidogrel administration longer. But if the patients have hemorrhagic risks, clopidogrel may be stopped early.4)7)16)18)21) Therefore, for preventing these risks of both hemorrhagic and ischemic complications, as well as for appropriate termination of clopidogrel administration, it is necessary to periodically follow up angiography and to closely observe clinical symptoms.19)
In our case, we experienced a delayed ischemic stroke at 27days after PED deployment. During the operation, no complications including thromboembolic event occurred and PED was appropriately deployed at the desired location. In post-operative angiography, the patency of AChA was well-maintained and the flow stagnation in aneurysm sac was arisen immediately after PED deployment. Despite the successful operation, we encountered an unexpected complication and the exact mechanism leading to ischemic stroke in our patients remains unclear. We suspect that the convex curvature of PED at AChA and PCoA induces a delayed occlusion of AChA. The convexity of PED made pores slightly spread and thereby tiny thrombus in the aneurysm sac could cross to the AChA through the enlarged pores. Another possibility is a gradual occlusion of AChA in the process of endothelial remodeling. Overgrowth of neo-intima around the aneurysm neck could be the cause of AChA occlusion.21) Intra-operatively, acute thromboembolic events can be detected easily and managed with glycoprotein IIb/IIIa platelet inhibitors; however, post-operatively, it is difficult to predict and reduce the complications. Therefore, in order to prevent thromboembolic events, it is important to observe neurologic changes both throughout and after the operation and to continue the dual antiplatelet therapy. The reported case suggest that, in order to prevent the occurrence of a delayed ischemic stroke, it is necessary to administer the dual antiplatelet therapy for over 3 months, as well as to check the follow-up angiography, and to closely observe the patient.
The flow-diverting device is an innovative technique allowing for a more effective and safe endovascular treatment of previously untreatable aneurysms; the outcomes of PED are also considerably better than the coil embolization. However, there are still complications, such as rupture of aneurysm, ischemic stroke, and flow diversion-induced ICH. In our case, despite the successful deployment of flow-diverting device, we observed a delayed ischemic stroke as an unexpected complication. Therefore, the neurosurgeon should be aware of the possibility of delayed complications, even in cases when no acute complications during the operation are observed. Furthermore, in order to prevent these delayed complications of the flow-diverting device, it is crucial to efficiently administer the dual antiplatelet therapy, as well as to check follow-up angiography and closely observe clinical symptoms.
Notes
References
1. Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. 2013; 2. 44(2):442–447. PMID: 23321438.

2. Chalouhi N, Tjoumakaris S, Starke RM, Gonzalez LF, Randazzo C, Hasan D, et al. Comparison of flow diversion and coiling in large unruptured intracranial saccular aneurysms. Stroke. 2013; 8. 44(8):2150–2154. PMID: 23723311.

3. Chitale R, Gonzalez LF, Randazzo C, Dumont AS, Tjoumakaris S, Rosenwasser R, et al. Single center experience with pipeline stent: feasibility, technique, and complications. Neurosurgery. 2012; 9. 71(3):679–691. PMID: 22653389.
4. Chiu AH, Cheung AK, Wenderoth JD, De Villiers L, Rice H, Phatouros CC, et al. Long-term follow-up results following elective treatment of unruptured intracranial aneurysms with the Pipeline embolization device. AJNR Am J Neuroradiol. 2015; 9. 36(9):1728–1734. PMID: 25999412.

5. Ding D, Starke RM, Liu KC. Microsurgical strategies following failed endovascular treatment with the pipeline embolization device: case of a giant posterior cerebral artery aneurysm. J Cerebrovasc Endovasc Neurosurg. 2014; 3. 16(1):26–31. PMID: 24765610.

6. Eller JL, Dumont TM, Sorkin GC, Mokin M, Levy EI, Snyder KV, et al. The Pipeline embolization device for treatment of intracranial aneurysms. Expert Rev Med Devices. 2014; 3. 11(2):137–150. PMID: 24506298.

7. Fiorella D, Hsu D, Woo HH, Tarr RW, Nelson PK. Very late thrombosis of a pipeline embolization device construct: case report. Neurosurgery. 2010; 9. 67(3 Suppl Operative):onsE313–onsE314. discussion onsE314PMID: 20679914.
8. Iosif C, Camilleri Y, Saleme S, Caire F, Yardin C, Ponomarjova S, et al. Diffusion-weighted imaging-detected ischemic lesions associated with flow-diverting stents in intracranial aneurysms: safety, potential mechanisms, clinical outcome, and concerns. J Neurosurg. 2015; 3. 122(3):627–636. PMID: 25559933.

9. Jeon HJ, Kim DJ, Kim BM, Lee JW. Pipeline embolization device for giant internal carotid artery aneurysms: 9-month follow-up results of two cases. J Cerebrovasc Endovasc Neurosurg. 2014; 6. 16(2):112–118. PMID: 25045651.

10. Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007; 8. 38(8):2346–2352. PMID: 17615366.

11. Kallmes DF, Hanel R, Lopes D, Boccardi E, Bonafé A, Cekirge S, et al. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol. 2015; 1. 36(1):108–115. PMID: 25355814.

12. Lall RR, Crobeddu E, Lanzino G, Cloft HJ, Kallmes DF. Acute branch occlusion after Pipeline embolization of intracranial aneurysms. J Clin Neurosci. 2014; 4. 21(4):668–672. PMID: 24156905.

13. McDonald RJ, McDonald JS, Kallmes DF, Lanzino G, Cloft HJ. Periprocedural safety of Pipeline therapy for unruptured cerebral aneurysms: Analysis of 279 Patients in a multihospital database. Interv Neuroradiol. 2015; 2. 21(1):6–10. PMID: 25934768.

14. Monteith SJ, Tsimpas A, Dumont AS, Tjoumakaris S, Gonzalez LF, Rosenwasser RH, et al. Endovascular treatment of fusiform cerebral aneurysms with the Pipeline Embolization Device. J Neurosurg. 2014; 4. 120(4):945–954. PMID: 24460489.

15. Nelson PK, Lylyk P, Szikora I, Wetzel SG, Wanke I, Fiorella D. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol. 2011; 1. 32(1):34–40. PMID: 21148256.

16. Nossek E, Chalif DJ, Chakraborty S, Lombardo K, Black KS, Setton A. Concurrent use of the Pipeline Embolization Device and coils for intracranial aneurysms: technique, safety, and efficacy. J Neurosurg. 2015; 4. 122(4):904–911. PMID: 25658781.

17. O'Kelly CJ, Spears J, Chow M, Wong J, Boulton M, Weill A, et al. Canadian experience with the pipeline embolization device for repair of unruptured intracranial aneurysms. AJNR Am J Neuroradiol. 2013; 2. 34(2):381–387. PMID: 22859284.
18. Piano M, Valvassori L, Quilici L, Pero G, Boccardi E. Midterm and long-term follow-up of cerebral aneurysms treated with flow diverter devices: a single-center experience. J Neurosurg. 2013; 2. 118(2):408–416. PMID: 23176329.

19. Saatci I, Yavuz K, Ozer C, Geyik S, Cekirge HS. Treatment of intracranial aneurysms using the pipeline flow-diverter embolization device: a single-center experience with long-term follow-up results. AJNR Am J Neuroradiol. 2012; 9. 33(8):1436–1446. PMID: 22821921.

20. Tan LA, Keigher KM, Munich SA, Moftakhar R, Lopes DK. Thromboembolic complications with Pipeline Embolization Device placement: impact of procedure time, number of stents and pre-procedure P2Y12 reaction unit (PRU) value. J Neurointerv Surg. 2015; 3. 7(3):217–221. PMID: 24553344.

21. Yeung TW, Lai V, Lau HY, Poon WL, Tan CB, Wong YC. Long-term outcome of endovascular reconstruction with the Pipeline embolization device in the management of unruptured dissecting aneurysms of the intracranial vertebral artery. J Neurosurg. 2012; 4. 116(4):882–887. PMID: 22264186.

Fig. 1
3D reconstruction of the left ICA angiography. A large PCoA aneurysm is located between PCoA and AChA (neck: 11 mm, length: 18 mm, width: 9 mm, postero-inferior projection). Proximal PCoA originates from the aneurysm neck, but proximal AChA is slightly separated from the aneurysm. ACoA and left MCA aneurysms are also visible (Black star: Large PCoA aneurysm, White arrow: AChA, Arrow head: PCoA). Lt = left; ICA = internal carotid artery; PCoA = posterior communicating artery; AChA = anterior choroidal artery; ACoA = anterior communicating artery; MCA = middle cerebral artery.
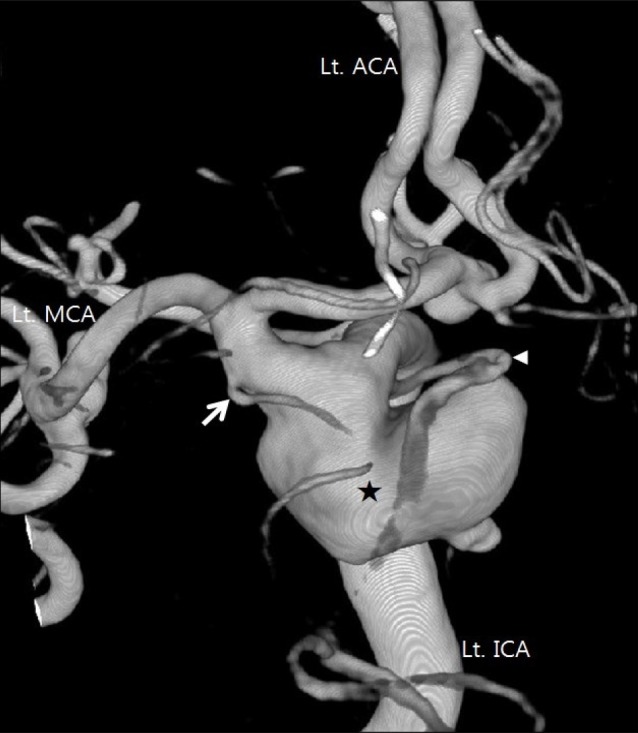
Fig. 2
(A, B) Post-operative right anterior oblique and lateral view. (C, D) 3D reconstruction of PED and coils. (C, D) PED is well-attached to the distal ICA wall (Black star: Large PCoA aneurysm, Arrow head: Deployed PED, White arrow: coil embolization of the left ACoA, MCA aneurysms, Black arrow: cranial fixator of a previous craniotomy, Black star: Large PCoA aneurysm). PED = Pipeline™ Embolization Device; ICA = internal carotid artery; PCoA = posterior communicating artery; ACoA = anterior communicating artery; MCA = middle cerebral artery.
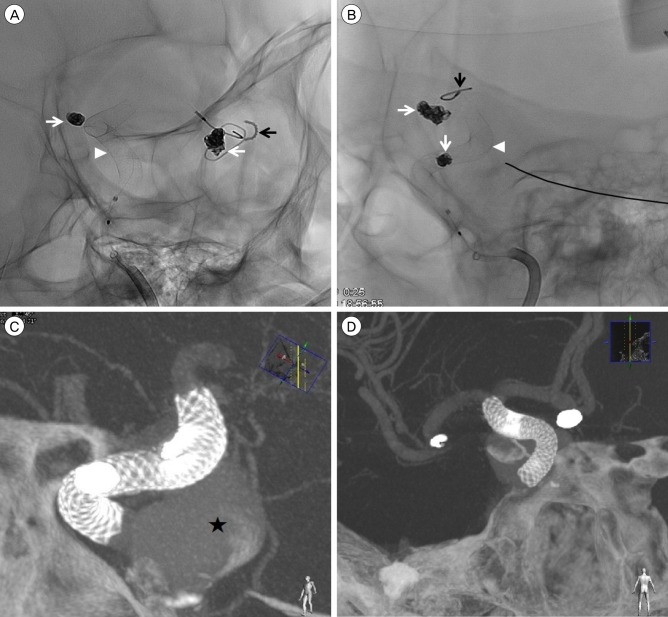
Fig. 3
(A) Post-operative angiographyin the arterial phase. After the deployment of PED, all of the branch vessels, including AChA and PCoA, are well-maintained. (B) Post-operative angiography in the venous phase. The flow stagnation at PCoA aneurysm sac is observed (White arrow: AChA, Arrow head: PCoA). PED = Pipeline™ Embolization Device; AChA = anterior choroidal artery; PCoA = posterior communicating artery.

Fig. 4
Diffusion MRI scan at 25 days after the endovascular treatment. The patient presented intermittent right hemiparesis, but there was no acute lesion in the territory of left AChA. MRI = magnetic resonance imaging; AChA = anterior choroidal artery.

Fig. 5
Diffusion MRI scan at 27 days after the endovascular treatment. The patient's hemiparesis was aggravated, so we rechecked diffusion MRI again. In follow-up MRI, acute ischemic stroke was found in the territory of the left AChA. MRI = magnetic resonance imaging; AChA = anterior choroidal artery.

Table 1
Complication rates of the flow-diverting device




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download