Abstract
Objective
We compared the effect of decompressive craniectomy between patients < 65 and ≥ 65 years age and investigated prognostics factors that may help predict favorable outcome in acute stroke patients undergoing decompressive surgery.
Materials and Methods
52 patients diagnosed with acute middle cerebral artery (MCA) territory infarction that underwent decompressive craniectomy were retrospectively reviewed. The outcome of all patients were evaluated by assessing the Glasgow coma scale, Glasgow outcome scale (GOS), and Modified Rankin scale (mRS) six months after the onset of the disease. 21 patients were preoperatively evaluated with a computed tomography angiography (CTA). Leptomeningeal collateral (LMC) circulation was graded using CTA by experienced neurosurgeons to assess its prognostic value.
Results
The thirty day mortality for patients ≥ 65 was 35.0% compared to 37.5% in patients < 65. There was no significant difference in the clinical and function outcome between the two groups (4.8 ± 1.2 vs. 4.5 ± 1.5, p = 0.474). Mortality was lower with early surgery (within 24 hours) group for both age groups (25% vs. 37.5% in ≥ 65, 20% vs. 40.7% in < 65). Longer intensive care units stay time and good collateral supply score were correlated with favorable outcome (p = 0.028, p = 0.018).
Conclusion
Decompressive craniectomy within 24 hours of stroke symptom onset improved survival in both the < 65 and ≥ 65 age groups. There was no significant difference in the functional outcome of both age groups. Unlike previous reports, old age, delayed operation, and multiple of infarct territories were not predictive of poor functional outcome. The presence of good collateral circulation may be a predictor of positive clinical outcome in acute ischemic stroke patients undergoing decompressive craniectomy.
Malignant cerebral infarction involving the middle cerebral artery (MCA) describes a condition in which severe progressive cerebral edema following the occlusion of the MCA leads to acute neurological deterioration. Such space occupying edema has been associated with a mortality rate as high as 80%.5) Decompressive craniectomy is a procedure performed in acute ischemic stroke patients in efforts to resolve fatal progressive edema and brain herniation. Studies have shown that an early craniectomy is associated with lower mortality and better functional recovery.14) Age, involvement of multiple vascular territories, and the presence of signs of herniation have also been considered as possible prognostic factors in acute ischemic stroke patients that underwent decompressive craniectomy. The status of leptomeningeal collateral (LMC) circulation has also been reported to be an independent predictor in the clinical outcome of acute ischemic stroke.10) Therefore, younger patients (≤ 60 years) that received surgical treatment within 24 hours have so far been associated with a more favorable outcome.2) Not many studies have reported the benefits of decompressive craniectomy in older patients (≥ 65). Decompressive craniectomy in the elderly has often been associated with higher mortality and poorer outcome, making it a procedure difficult to recommend. The authors have compared and analyzed the clinical outcomes and possible prognostic factors, including the value of collateral circulation, in patients up to the age of 80 that underwent decompressive craniectomy for treatment of acute MCA infarction.
From January 2008 to December 2014 a total of 52 patients underwent decompressive craniectomy. The patients were retrospectively reviewed using our institute's electronic medical records database. Of the 52 patients included in the study, 32 patients were below the age of 65 and 20 were of age 65 and older.
The surgical inclusion criteria were : (i) infarction involving > 50% of the MCA territory measured in computed tomography (CT) scan and/or magnetic resonance imaging (MRI), with an acute onset of symptoms corresponding to the ischemic lesion; (ii) development of neurologic deficits such as progressive motor weakness, pupil abnormalities, and deterioration of consciousness.
All patients included in the study underwent a large ipsilateral craniectomy and augmentative duraplasty. The craniectomy was performed in efforts to obtain a bone flap greater than 14 cm (anteroposterior diameter) × 10 cm (superoinferior diameter) to provide sufficient decompression. The dura was opened in a stellate fashion for decompression and the duraplasty was performed with a collagen based artificial dura of bovine origin (Lyoplant®, Aesculap, PA, USA). We chose to perform large craniectomies based on previous studies that suggest bone flaps larger than 14 cm may be associated with better outcome.3)15)
Out of the 52 patients, 21 patients were preoperatively evaluated with a computed tomography angiography (CTA) within 4 hours of the onset of stroke symptoms. LMCs were evaluated and graded independently by a staff neurosurgeon and a neurosurgical fellow using a collateral supply score (CS) between 0-3 (Fig. 1). A score of zero indicated that there was no collateral supply to the occluded MCA territory. A score of 1 indicated collateral supply filling greater 0% but less than 50% of the occluded MCA territory. A score of 2 indicated collateral supply filling greater than 50% less than 100% of the occluded MCA territory. A score of 3 was assigned to 100% collateral supply to the occluded MCA territory. Patients with a collateral score ≥ 2 were considered to have good collateral circulation.
The clinical status of all patients were recorded at admission and preoperatively using the Glasgow coma scale (GCS). National Institutes of Health Stroke Scale (NIHSS) upon admission to the emergency room was also noted. The clinical and functional outcome of all patients were evaluated by assessing the GCS, Glasgow outcome scale (GOS), and Modified Rankin scale (mRS) six months after the onset of the disease. mRS score between zero to four was considered favorable.
All statistical data were analyzed using SPSS Version 19 (SPSS Inc., Chicago, IL, USA). Quantitative data are shown as mean ± standard deviation (SD). Patients were analyzed by dichotomizing the six month mRS score into favorable (mRS score, ≤ 4) versus poor outcome (mRS score, > 4). Patient data was also dichotomized by age (Patients < 65 vs. Patients ≥ 65) for comparative analysis. Comparison of values was performed by independent two sample t-test and χ2 test, and logistic regression analysis was used to analyze the influence of pretreatment variables on prognosis of the patients. A p-value of 0.05 or less was considered significant.
The mean age of patients that received decompressive craniectomy was 57.7 ± 12.6 years (range: 24-80) and the ratio of males to females was 1.17:1. The mean preoperative GCS and NIHSS score was 12.9 ± 2.0 and 16.1 ± 4.7, respectively. Sole MCA territory infarction was observed in 30 patients. Internal carotid artery (ICA) occlusion leading to anterior cerebral artery (ACA) and MCA territory infarction was found in 21 patients. One patient was diagnosed with MCA and posterior cerebral artery (PCA) territory infarction. In 26 patients (50.0%), tissue plasminogen activator (tPA) was indicated and used. The mean time to operation from the onset of symptoms was 55.6 ± 69.8 h (range: 8-416), and nine patients received decompressive craniectomy within 24 hours. The mean intensive care unit (ICU) stay time was 17.8 ± 11.8 days. The average preoperative midline shifting was 10.6 ± 4.1 mm, and nine patients showed preoperative brain stem compression in the preoperative brain CT. Out of the 22 patients that were preoperatively evaluated with a CTA, nine patients displayed collateral supply score ≥ 2.
The thirty day mortality of all patients that underwent decompressive craniectomy was 38.4% (18 patients), and the one year mortality was 40.3% (20 patients). The mean GCS of the survivors at six months was 11.9 ± 2.6. The mean six month mRS and GOS of the survivors were 3.61 ± 0.9 and 3.03 ± 0.51, respectively. The thirty day mortality of patients that underwent early decompression (within 24 hours) was 22.2% (2 out of 9 patients), and the mortality of those that underwent surgery after 24 hours was 40.4% (17 out of 42 patients). The mean six month mRS and GOS of the survivors that underwent decompressive craniectomy within 24 hours were 4.0 ± 0.58 and 2.86 ± 0.38, respectively. The mean six month mRS and GOS of the survivors that received surgery after 24 hours were 3.55 ± 0.93 and 3.04 ± 0.54, respectively.
A sum of 20 patients ≥ 65 years of age received decompressive surgery. The thirty day mortality for these patients was 35.0% (7 patients) and one year mortality was 40.0% (8 patients). Five patients expired due to complications unrelated to cerebral edema. Three of the patients expired due to complications of pneumonia, one patient suffered non ST-segment elevation myocardial infarction, and one patient passed away from wound infection associated sepsis five months after the surgery. The mean GCS of the survivors at six months was 13.2 ± 2.0. The mean six month mRS and GOS of the survivors was 3.91 ± 0.67 and 3.25 ± 0.75, respectively. The thirty day mortality of elderly patients that underwent decompressive craniectomy within 24 hours was 25.0% (1 out of 4 patients), and the mortality of those that underwent surgery after 24 hours was 37.5% (6 out of 16 patients). This is comparable to the mortality of the younger group which was 20.0% (1 out of 5 patients) for early surgery and 40.7% (11 out of 27) for delayed surgery.
A total of 27 patients showed favorable outcome (mRS ≤ 4). Factors associated with poor outcome (mRS ≥ 5) were evidence of preoperative stem compression (p = 0.007) and poor collateral supply score (p = 0.007), as shown in Table 2. Unlike previous reports, shorter ICU stay time (p = 0.040) was also associated with poor outcome. Older age, longer time to operation (> 24 hrs), and involvement of multiple vascular territories were not significantly associated with poor outcome. Univariate linear regression analysis supported that patients with preoperative stem compression and a low collateral score were at higher risk for poor prognosis than those who did not (Table 3). The multivariate analysis showed that patients with shorter ICU stay were at risk for poor prognosis. Unfortunately, due to the small number of patients that received preoperative CTA, collateral circulation score could not be included in the multivariate analysis.
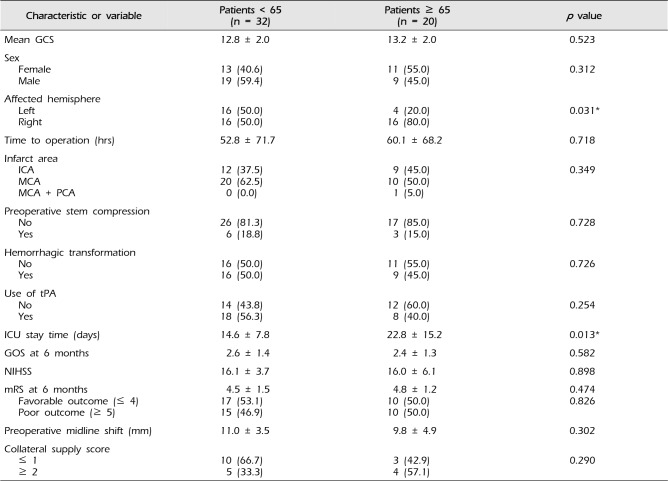
Comparative analysis of patients younger than the age of 65 and those of age 65 and older was performed (Table 4). There were no significant differences in the mean preoperative GCS and NIHSS, time to operation, presence of hemorrhagic transformation or preoperative stem compression, and mean preoperative midline shifting. The mean GOS and mRS of the two groups at six months and collateral supply score did not show statistical differences. Right hemisphere involvement was significantly more abundant (p = 0.031) and ICU stay time was also significantly longer (p = 0.013) in the age ≥ 65 group.
MCA territory ischemia accounts for about 10-15% of all supratentorial infarctions. Mortality rates have been reported to be as high as 80% and leave those that survive with severe disabilities. Despite medical advancements, the management of progressive malignant edema is very challenging. The role of decompressive craniectomy in acute MCA infarctions has been reported to be lifesaving and improve functional outcome in recent prospective, randomized studies.14)16) Many studies have also been carried out to determine the possible prognostic factors associated with favorable outcome of decompressive craniectomy in acute MCA infarction. The current consensus of these studies seems to be that younger patients (< 60) with better initial GCS that undergo surgery within 24 hours of ictus before clinical presentation of cerebral herniation show a significantly more favorable outcome.2)7)9)
Ischemic stroke, however, is a disease that is directly related to age, and the prevalence of ischemic stroke has been reported to nearly double in patients over the age of 65 among all ethnicities.8) As more countries become aged, it is no surprise that recent studies have therefore been focused on the effect of decompressive craniectomy for acute ischemic stroke in elderly patients. The Decompressive Surgery for the Treatment of Malignant Infarction of the Middle Cerebral Artery (DESTINY) II trial which compared the outcome of the patients over the age of 60 that underwent decompressive craniectomy to those that were conservatively managed, concluded that both survival and functional outcome was significantly improved in the decompressive craniectomy group.6) In the current series, many of the findings coincided with previous studies, yet we also noticed some striking disparities.
The overall one year mortality turned out to be 40.3% which is comparable to the previous reports ranging from 8-50%.1) The mortality of those that underwent decompressive craniectomy within 24 hours was much lower (22.2% vs. 40.4%) than those that received delayed surgery, which also coincides with previous studies.13) The functional outcome represented by the mean six month mRS and GOS, however, was not significantly improved in the early decompressive craniectomy group. Upon closer inspection, the distribution of functional outcome, as seen in Fig. 2, is quite different between the two groups. The early decompressive craniectomy group showed a greater percentage of patients in the favorable outcome category (mRS ≤ 4), but the majority of these patients (five out of nine) had a mRS of 4 (immobile and dependent). The delayed decompressive craniectomy group had a lower percentage of patients with favorable outcome as suggested by previous studies, but had a greater percentage of patients with mRS 3 or less (disabled but independent). This lack of statistically significant difference in the mean functional outcome score and reversal of distribution in the mRS between the two groups may mostly be attributed to the small population included in the early surgery group, which may not be representative of those that receive decompressive craniectomy within 24 hours. Another explanation may be that the patients that displayed severe symptoms and neurologic deficit at initial presentation were more quickly acted upon to receive surgical intervention. This explanation may be supported by the fact the mean NIHSS of those that received early decompressive craniectomy was relatively higher than the mean NIHSS value of those that received delayed surgery (18.1 vs. 15.6).
The 30 day mortality of the elderly patients (35.0%) was also comparable to previously reported data and to that of our younger group (37.5%). However, only one patient (3%) in the < 65 group passed away within 30 days due to causes unrelated to the cerebral infarction (sudden cardiac arrest of unknown origin), while three out of 20 (15%) elderly patients expired from complications of pneumonia and myocardial infarction. Additionally, an elderly patient passed away five months after the surgery due to complications of delayed surgical site infection. While decompressive craniectomy provides an opportunity for survival among all ages of patients, it is evident that older patients are more frequently associated with deaths resulting from complications of underlying disease or other unrelated reasons such as infection despite undergoing decompressive craniectomy. In terms of functional outcome, the mRS distribution of the two age groups (Fig. 3) shows that while a similar percentage of patients fall in the favorable outcome category, a greater percentage of patients in the < 65 group have a mRS ≤ 3. This distribution of functional outcome is similar to that of the results reported by the DESTINY II trial in which about 6% of subjects > 60 years old who received decompressive craniectomy had a mRS of < 4 at 12 months versus 43% in the younger group.6) Therefore, when faced with the challenge of treating a disease that leaves so many disabled and dependent, not only should the treatment be tailored to best benefit the patient, but treatment options and prognosis should also be discussed in full with the caregivers. In support of surgical decompression, a systemic review conducted by Rahme et al. revealed that despite being left with moderately severe or severe disability, most patients and caregivers expressed satisfaction with life and would retrospectively consent to undergoing surgery.12)
In order to provide a better prediction of prognosis and to help in the decision making of treatment for physicians, several variables were analyzed for prognostic value. Unlike previous studies, older age, longer time to operation (> 24 hrs), and involvement of multiple vascular territories were not suggestive of poor outcome. Preoperative stem compression suggesting brain herniation or imminent herniation has already been reported to be associated with poor prognosis.2) All but one patient in this series expired when preoperative CT revealed brain stem compression. In a novel attempt to include collateral circulation as a possible prognostic factor in decompressive craniectomy patients, we found that patients with collateral supply score ≥ 2 had significantly better functional outcome than those with low scores. A good collateral status has been associated with reduced infarction size, higher recanalization rates, and major reperfusion.10)11) We believe that decompressive craniectomy not only allows for better preservation of collateral vessels, but also provides a better chance for recanalization in those that have a good collateral supply. From our experience of performing decompressive craniectomy in stroke patients, it was also evident that craniectomies performed before the progression of edema and midline shifting allowed the surgeon to better preserve cortical structure and perform duraplasty with more ease. Therefore, early craniectomy should strongly be considered before the progression of cerebral edema leading to the obliteration of the collateral vessels.
One of the odd findings of this study was that shorter ICU stay time was associated with poor outcome. We believe that this data needs to understood by taking into account that the poor prognosis group includes the patients that passed away (mRS = 6). Many of these patients in our series died within one week (range: 4-42 days), while the patients that survived stayed a minimum of eight days (range: 8-60 days).
Comparative analysis of the two age groups revealed that the length of ICU stay was longer and right hemisphere involvement was more common in the elderly group. The longer ICU stay time in the elderly can mostly be attributed to the longer time it took for weaning of mechanical ventilation and the management of comorbities such as pneumonia. Right hemisphere infarction has been known to present with lower NIHSS scores than the actual lesion volume may suggest.4) We believe elderly patients included in this study showed more right hemisphere involvement because patients and caregivers were more willing to proceed with aggressive treatment such as decompressive craniectomy when initial symptoms are less severe or subtle, as is in right hemisphere infarction, in pursuit of better functional recovery.
There are several limitations to this study. First of all, it is retrospective in nature and was carried out in a single center. Some populations may not have been well represented due to the relatively small cohort of patients. This pertains especially to the population of patients that received early decompressive craniectomy. With the support of many published studies, we hope to produce better data by more actively providing indicated patient with the opportunity to receive early surgery in the future. CTA was carried out in less than half of the population included in the study and therefore could not be included for multivariate analysis. Additional data is currently being collected and analyzed to make a strong correlation. We also believe that a follow up CTA would have been helpful in supporting our idea that decompressive craniectomy can help in preserving collateral circulation and providing a better opportunity for recanalization. Additionally, selection bias may have been introduced as a result of family-member involvement in the decision-making of treatments.
The current study shows that early decompressive craniectomy improves overall survival among all age groups. In terms of functional outcome, more patients in the < 65 age group were able to achieve an independent functional status. Our study also shows that the evaluation of collateral circulation can be a helpful tool in predicting clinical outcome in acute ischemic stroke patients undergoing decompressive craniectomy. Therefore, we believe that early decompressive craniectomy is a modality of treatment that should be recommended among all ages of patients diagnosed with acute MCA infarction.
References
1. Agarwalla PK, Stapleton CJ, Ogilvy CS. Craniectomy in acute ischemic stroke. Neurosurgery. 2014; 2. 74(Suppl 1):S151–S162. PMID: 24402484.

2. Chen CC, Cho DY, Tsai SC. Outcome of and prognostic factors for decompressive hemicraniectomy in malignant middle cerebral artery infarction. J Clin Neurosci. 2007; 4. 14(4):317–321. PMID: 17275311.

3. Curry WT Jr, Sethi MK, Ogilvy CS, Carter BS. Factors associated with outcome after hemicraniectomy for large middle cerebral artery territory infarction. Neurosurgery. 2005; 4. 56(4):681–692. discussion 681-92. PMID: 15792506.

4. Fink JN, Selim MH, Kumar S, Silver B, Linfante I, Caplan LR, et al. Is the association of National Institutes of Health Stroke Scale scores and acute magnetic resonance imaging stroke volume equal for patients with right- and left-hemisphere ischemic stroke? Stroke. 2002; 4. 33(4):954–958. PMID: 11935043.

5. Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. 'Malignant' middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996; 4. 53(4):309–315. PMID: 8929152.
6. Juttler E, Unterberg A, Woitzik J, Bosel J, Amiri H, Sakowitz OW, et al. Hemicraniectomy in older patients with extensive middle-cerebral-artery stroke. N Engl J Med. 2014; 3. 370(12):1091–1100. PMID: 24645942.

7. Kilincer C, Asil T, Utku U, Hamamcioglu MK, Turgut N, Hicdonmez T, et al. Factors affecting the outcome of decompressive craniectomy for large hemispheric infarctions: a prospective cohort study. Acta Neurochir (Wien). 2005; 6. 147(6):587–594. discussion 594. PMID: 15739038.

8. Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, et al. Stroke in a biracial population: the excess burden of stroke among blacks. Stroke. 2004; 2. 35(2):426–431. PMID: 14757893.
9. Koh MS, Goh KY, Tung MY, Chan C. Is decompressive craniectomy for acute cerebral infarction of any benefit? Surg Neurol. 2000; 3. 53(3):225–230. PMID: 10773253.

10. Maas MB, Lev MH, Ay H, Singhal AB, Greer DM, Smith WS, et al. Collateral vessels on CT angiography predict outcome in acute ischemic stroke. Stroke. 2009; 9. 40(9):3001–3005. PMID: 19590055.

11. Miteff F, Levi CR, Bateman GA, Spratt N, McElduff P, Parsons MW. The independent predictive utility of computed tomography angiographic collateral status in acute ischaemic stroke. Brain. 2009; 8. 132(Pt 8):2231–2238. PMID: 19509116.

12. Rahme R, Zuccarello M, Kleindorfer D, Adeoye OM, Ringer AJ. Decompressive hemicraniectomy for malignant middle cerebral artery territory infarction: is life worth living? J Neurosurg. 2012; 10. 117(4):749–754. PMID: 22920962.

13. Schwab S, Steiner T, Aschoff A, Schwarz S, Steiner HH, Jansen O, et al. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke. 1998; 9. 29(9):1888–1893. PMID: 9731614.

14. Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007; 3. 6(3):215–222. PMID: 17303527.

15. Walz B, Zimmermann C, Bottger S, Haberl RL. Prognosis of patients after hemicraniectomy in malignant middle cerebral artery infarction. J Neurol. 2002; 9. 249(9):1183–1190. PMID: 12242536.

16. Zhao J, Su YY, Zhang Y, Zhang YZ, Zhao R, Wang L, et al. Decompressive hemicraniectomy in malignant middle cerebral artery infarct: a randomized controlled trial enrolling patients up to 80 years old. Neurocrit Care. 2012; 10. 17(2):161–171. PMID: 22528280.

Fig. 1
Collateral supply status measured by leptomeningeal collateral grade in CTA. (A) CS = 0, (B) CS = 1, (C) CS = 2, (D) CS = 3. CTA = computed tomography angiography; CS = collateral supply.
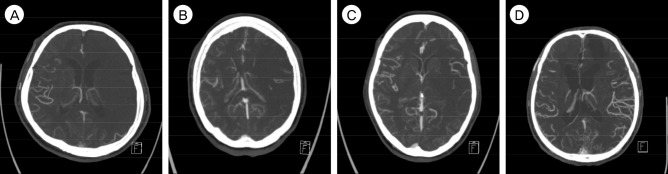
Fig. 2
Distributions of modified Rankin scale score in patient that underwent early (≤ 24 hours) decompressive craniectomy and delayed (> 24) decompressive craniectomy.
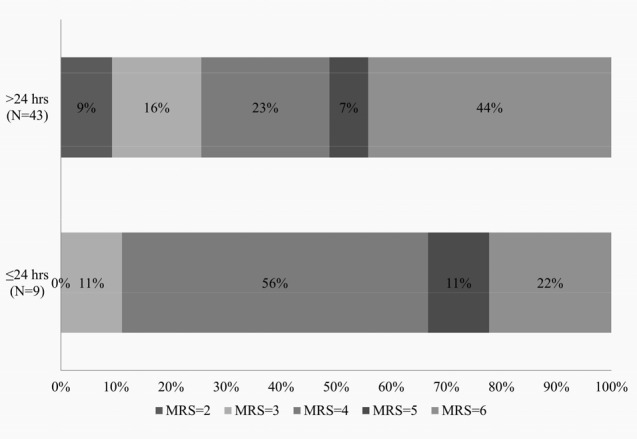
Fig. 3
Distribution of modified Rankin scale score in decompressive craniectomy patients aged ≥ 65 and < 65.
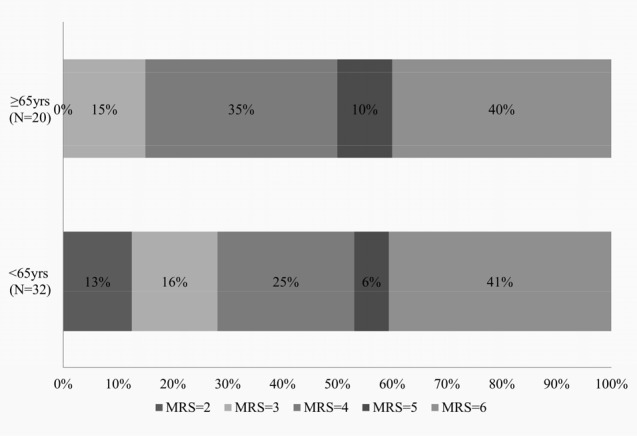
Table 1
Baseline characteristics of 52 patients that underwent decompressive craniectomy
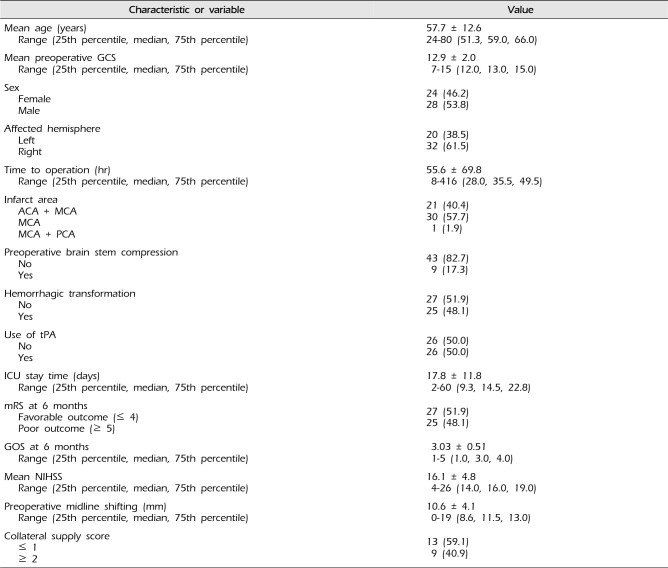
Table 2
Comparison of 52 patients that underwent decompressive craniectomy by modified Rankin scale
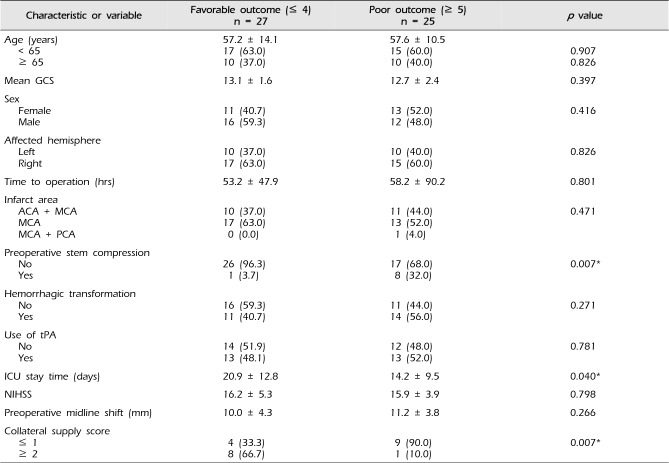
Table 3
Logistic regression analysis for modified Rankin scale > 4 model
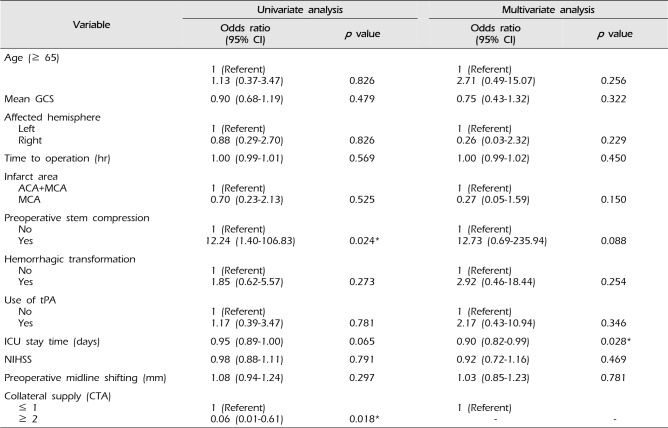
Table 4
Comparison of 52 patients that underwent decompressive craniectomy by age
Values are presented as number (%) or standard deviation.
GCS = Glasgow coma scale; ACA = anterior cerebral artery; MCA = middle cerebral artery; PCA = posterior cerebral artery; tPA = tissue plasminogen activator; ICU = intensive care unit; GOS = Glasgow outcome scale; NIHSS = National Institutes of Health Stroke Scale; mRS = modified Rankin scale




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download