Abstract
A giant serpentine aneurysm (GSA) in the anterior cerebral artery (ACA) poses a technical challenge in treatment given its large size, unique neck, and dependent distal vessels. Here we report the case of a GSA in the ACA successfully treated with a combined surgical and endovascular approach. A 54-year-old woman presented with dull headache. On brain computed tomography (CT), a large mass (7 cm × 5 cm × 5 cm) was identified in the left frontal lobe. Cerebral angiography revealed a GSA in the left ACA. Bypass surgery of the distal ACA was performed, followed byocclusion of the entry channel via an endovascular approach. Follow-up CT performed 5 days after treatment revealed disappearance of the vascular channel and peripheral rim enhancement. Follow-up imaging studies performed 7 months after treatment revealed gradual reduction of the mass effect and patency of bypass flow. No complications were noted over a period of 1 year after surgery.
A giant serpentine aneurysm (GSA) was first described by Segal and McLaurin in 1977.15) AGSA has been defined as a partially thrombosed aneurysm (larger than 25 mm in diameter), with tortuous vascular channels that have separate entry and exit pathways. It has various symptoms, and the mass effect is the most common.2)5)16) A GSA has a poor prognosis,16) and it can grow rapidly if not treated promptly. It frequently occurs in the middle cerebral artery,5) and its occurrence in the anterior cerebral artery (ACA) is extremely rare. A GSA in the ACA poses a technical challenge in treatment given its large size, unique neck, and dependent distal vessels. Here we report the case of a GSA in the ACA successfully treated with a combined surgical and endovascular approach.
A 54-year-old woman presented with dull headache for 3 months. On brain computed tomography (CT), a large mass was identified in the left frontal lobe (Fig. 1A, B). Cerebral angiography revealed a GSA in the left ACA (Fig. 2). Because of the tortuosity of the cerebral artery and the possibility of ischemic injury to perforating arteries, balloon test occlusion was not performed. She was treated with in situ "side-to-side" anastomosis of the distal ACA, followed by occlusion of the entry channel via an endovascular approach using detachable coils (Fig. 3B). The local ethics committee approved the retrospective use of the patient's data.
Bypass was performed via a unilateral interhemispheric approach (Fig. 3A). A small craniotomy (4 × 4 cm) was performed at the right frontal bone, including the superior sagittal sinus. A U-shaped incision was then made in the dura, followed by arachnoid dissection of the parasagittal area. After locating the genu of the corpus callosum, a pair of distal ACAs believed to be pericallosal arteries was located just above the genu, and in situ "side-to-side" anastomosis was performed. The patency of anastomosis was confirmed using micro-Doppler examination.
One day after bypass surgery, transient right hemiparesis was noted. However, she eventually recovered. Thromboembolic infarction was not detected on magnetic resonance imaging (MRI), and cerebral angiography revealed patency of in-situ bypass flow. Four days after bypass surgery, endovascular occlusion of the GSA entry channel was performed. Additionally, right internal carotid artery (ICA) angiography was performed under general anesthesia, and it revealed patency of in-situ bypass flow (Fig. 3E). A 6F Envoy guiding catheter (Codman, Raynham, MA, USA) was introduced into the left ICA, and endovascular occlusion was initiated.
The perforating artery was located in the entry channel of the GSA. It is believed to be associated with the lenticulostriate artery; therefore, it should not be sacrificed. The inner lumen of the GSA was gradually widened distally; however, the lumen suddenly narrowed. Occlusion of only the entry channel appeared difficult. Furthermore, the starting point of the GSA was not clear. Therefore, endovascular occlusion was divided into 2 steps (Fig. 3B). After setting the working angle, an Echelon-10 microcatheter (eV3 Neurovascular, Inc., Irvine, CA, USA) was advanced in the lumen of the GSA. In the initial step, the widest part of the GSA distally was loosely packed using large coils. In the next step, using this packing as a support, the proximal narrow part of the GSA between the distal perforator of the entry channel and the beginning of the aneurysm was densely packed using coils. After occlusion of the entry channel, left ICA angiography revealed exclusion of the GSA, collateral flow via bypass, and retrograde contrast filling of the GSA (Fig. 4).
Follow-up CT performed 5 days after treatment revealed disappearance of the vascular channel and peripheral rim enhancement. Her modified Rankin Scale score at discharge was 0. Follow-up imaging studies performed 4 and 7 months after treatment revealed gradual reduction of the mass effect and patency of bypass flow (Fig. 1C-E). No complications were noted over a period of 1 year after surgery.
We reported the case of a GSA in the ACA successfully treated with a combined approach. The postsurgical outcomes of patients with a GSA have been poor. In a review of the literature, a morbidity rate of up to 33.5% was noted.2)7) This high morbidity rate may be associated with the characteristics of a GSA. First, a GSA has an indefinite neck. The exiting channel of an aneurysm supplies normal brain parenchyma. Therefore, a perioperative stroke frequently occurs. In order to improve this, consideration of collateral circulation is mandatory. Before treatment, the bypass technique should be considered. Second, the size of the aneurysm is more than 25 mm. Such a large size can result in parenchymal damage during dissection and debulking. Interestingly, after occlusion of GSA flow, reductions in the mass effect and edema have been frequently reported,3)13)16) and these reductions have been reported even after occluding the exiting channel.9) Considering these findings, total resection of the mass may not be required. Reconstruction procedures, such as bypass with an exit channel, should be considered before treatment. In summary, before treatment, it is necessary to determine whether only deconstruction treatment would be sufficient. If reconstruction is performed, the essential occlusion site, treatment modality, number of operation stages, and priority should be considered. All decisions should be individualized.
ACA bypass for a GSA is believed to be "challenging."1) The in situ "side-to-side" bypass technique for ACA bypass was first reported in 1986.10) Because this technique is simple, has less restriction, and is physiologically ideal, it has been the most commonly used technique in the ACA territory.6)8)11) A small surgical wound is beneficial to patients if it does not interfere with the operation. However, this approach should only be considered if the operational risk does not increase. The surgical field for bilateral distal ACA bypass is sufficiently secured under the inferior margin of the falx. Therefore, a unilateral interhemispheric approach via a small craniotomy during in situ bypass for a lesion is feasible.12) However, craniotomy should be wide if trapping of the aneurysm is considered.
Endovascular therapy has an important role in the treatment of GSAs.13)14) As a GSA usually has a complex structure, surgery is frequently needed along with endovascular therapy. In the combination of bypass and endovascular occlusion, the time interval between bypass and endovascular occlusion should be carefully considered. It might be better to perform endovascular occlusion promptly if the patient is stable.
In the present case, transient ischemic attack occurred one day after surgery. We confirmed the condition of the patient and the patency of bypass using MRI and cerebral angiography. After 3 days of care and monitoring, proximal occlusion was performed. In this case, the outlet of the GSA was not occluded. Therefore, blood flow via bypass might affect the growth of the GSA. However, the distal ACA has low blood flow similar to superficial temporal artery. Thus, the effect of backflow on the aneurysm is minimal.1)4) Our case indicates the stability of proximal occlusion.
Simple surgery may reduce perioperative morbidity. For good results, the surgeon should choose the easiest and most appropriate procedures. It is not necessary to only use a single technique. In situ bypass via a unilateral anterior interhemispheric approach and endovascular proximal occlusion are technically easy procedures. In the present case, selection of the easiest and most appropriate procedures allowed for good results.
References
1. Abla AA, Lawton MT. Anterior cerebral artery bypass for complex aneurysms: An experience with intracranial-intracranial reconstruction and review of bypass options. J Neurosurg. 2014; 6. 120(6):1364–1377. PMID: 24745711.

2. Aletich VA, Debrun GM, Monsein LH, Nauta HJ, Spetzler RF. Giant serpentine aneurysms: A review and presentation of five cases. AJNR Am J Neuroradiol. 1995; 5. 16(5):1061–1072. PMID: 7639128.
3. Amin-Hanjani S, Chen PR, Chang SW, Spetzler RF. Long-term follow-up of giant serpentine MCA aneurysm treated with EC-IC bypass and proximal occlusion. Acta Neurochir (Wien). 2006; 2. 148(2):227–228. PMID: 16322902.

4. Carlson AP. Tailored PICA revascularization for unusual ruptured fusiform vertebro-PICA origin aneurysms: Rationale and case illustrations. J Neurol Surg Rep. 2015; 11. 76(2):e275–e278. PMID: 26623241.

5. Christiano LD, Gupta G, Prestigiacomo CJ, Gandhi CD. Giant serpentine aneurysms. Neurosurg Focus. 2009; 5. 26(5):E5.

6. Dunn GP, Gerrard JL, Jho DH, Ogilvy CS. Surgical treatment of a large fusiform distal anterior cerebral artery aneurysm with in situ end-to-side A3-A3 bypass graft and aneurysm trapping: Case report and review of the literature. Neurosurgery. 2011; 2. 68(2):E587–E591. discussion E591. PMID: 21135720.
7. Fanning NF, Kelleher MO, Ryder DQ. The pretzel sign: Angiographic pattern of tortuous intra-aneurysmal blood flow in a giant serpentine aneurysm. Br J Neurosurg. 2003; 2. 17(1):67–71. PMID: 12779205.

8. Ferroli P, Ciceri E, Addis A, Broggi G. Self-closing surgical clips for use in pericallosal artery-pericallosal artery side-to-side bypass. J Neurosurg. 2008; 8. 109(2):330–334. PMID: 18671649.

9. Horowitz MB, Yonas H, Jungreis C, Hung TK. Management of a giant middle cerebral artery fusiform serpentine aneurysm with distal clip application and retrograde thrombosis: Case report and review of the literature. Surg Neurol. 1994; 3. 41(3):221–225. PMID: 8146737.

10. Ikeda A, Shibuya M, Okada T, Kageyama N. Microvascular side-to-side anastomosis. basic problems and clinical applications. Neurol Med Chir (Tokyo). 1986; 5. 26(5):379–384. PMID: 2429217.
11. Lawton MT, Hamilton MG, Morcos JJ, Spetzler RF. Revascularization and aneurysm surgery: Current techniques, indications, and outcome. Neurosurgery. 1996; 1. 38(1):83–92. discussion 92-4. PMID: 8747955.

12. Lehecka M, Dashti R, Hernesniemi J, Niemela M, Koivisto T, Ronkainen A, et al. Microneurosurgical management of aneurysms at A3 segment of anterior cerebral artery. Surg Neurol. 2008; 8. 70(2):135–151. discussion 152. PMID: 18482754.

13. Otsuka G, Miyachi S, Handa T, Negoro M, Okamoto T, Suzuki O. Endovascular trapping of giant serpentine aneurysms by using guglielmi detachable coils: Successful reduction of mass effect. report of two cases. J Neurosurg. 2001; 5. 94(5):836–840. PMID: 11354420.
14. Park ES, Ahn JS, Park JC, Kwon DH, Kwun BD, Kim CJ. STA-ACA bypass using the contralateral STA as an interposition graft for the treatment of complex ACA aneurysms: Report of two cases and a review of the literature. Acta Neurochir (Wien). 012; 8. 154(8):1447–1453. PMID: 22692589.

15. Segal HD, McLaurin RL. Giant serpentine aneurysm. report of two cases. J Neurosurg. 1977; 1. 46(1):115–120. PMID: 830809.
16. Suzuki S, Takahashi T, Ohkuma H, Shimizu T, Fujita S. Management of giant serpentine aneurysms of the middle cerebral artery--review of literature and report of a case successfully treated by STA-MCA anastomosis only. Acta Neurochir (Wien). 1992; 117(1-2):23–29. PMID: 1514425.
Fig. 1
(A, B) Initial brain computed tomography images showing a heterogeneous large mass (7 cm × 5 cm × 5 cm) with central and rim enhancements (A: non-enhanced axial image; B: enhanced axial image). (C) Five days after treatment. Follow-up brain computed tomography axial view showing disappearance of the vascular channel and peripheral rim enhancement. (D) Four months after treatment.Axial view showing reduction of the mass effect. (E) Seven months after treatment. Axial view showing further reduction of the mass effect.
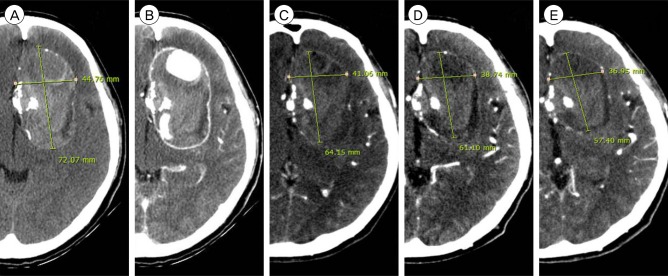
Fig. 2
Internal carotid artery angiography images. (A) Anteroposterior view in the arterial phase showing a giant serpentine aneurysm (GSA) and filling defect of the left distal anterior cerebral artery (ACA) territory (black asterisk: left distal ACA filling defect; white asterisk: GSA lumen with contrast filling). (B) Lateral view in the arterial phase showing a GSA and entry channel (white arrow: entry point of the GSA). (C) Lateral view in the late venous phase showing delayed filling of the left distal ACA territory (small black arrows: territory of the left distal ACA). (D) Oblique view in the late venous phase showing the exit channel of the GSA (black arrow: exit channel of the GSA).
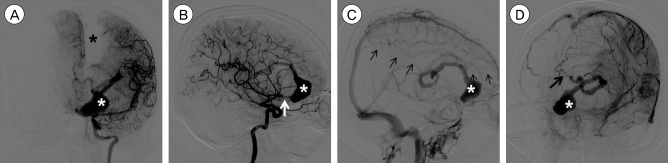
Fig. 3
(A) The condition after bypass and before temporary clip removal. (B) Schematic drawing of the treatment plan for the giant serpentine aneurysm (GSA) in the distal anterior cerebral artery (ACA). α: In situ "side-to-side" bypass of both distal ACAs, β: Occlusion of the entry channel via an endovascular approach using detachable coils. Occlusion is performed in 2 steps. First, the distal widest part of the GSA is loosely packed using large coils (green helix). Second, the proximal narrow part between the distal perforator of the feeding artery and the beginning of the aneurysm is densely packed using coils (blue helix).
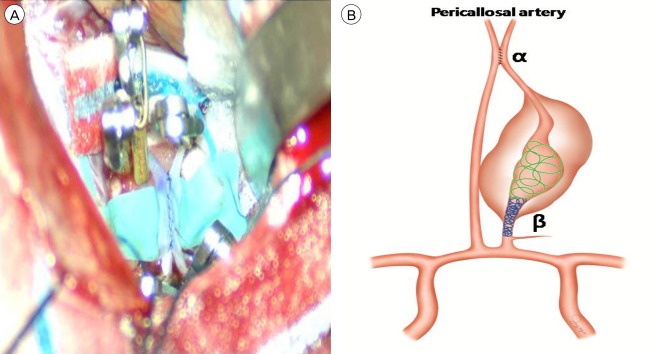
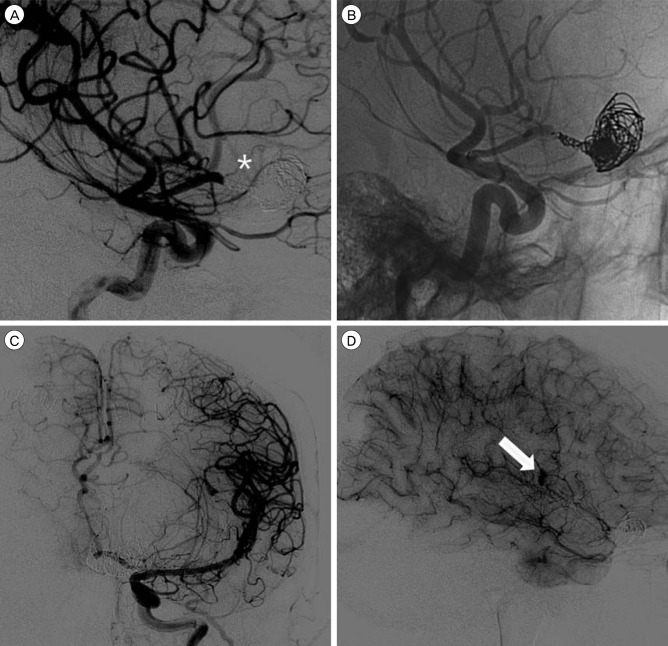
Fig. 4
(A) Lateral view of the working angle after endovascular occlusion showing that the perforating artery is not sacrificed (asterisk indicates the perforating artery). (B) Native view of the working angle showing a loosely packed distal part and densely packed proximal part of thegiant serpentine aneurysm (GSA). (C) Left internal carotid artery angiography in the late arterial phase (anteroposterior view) after entry channel occlusion showing a GSA and patient collateral flow via bypass. (D) Left internal carotid artery angiographyin the capillary phase (lateral view) after entry channel occlusion showing retrograde contrast filling of the GSA (the white arrow indicates retrograde contrast filling).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download