Abstract
Simultaneous or subsequent bilateral thalamic hemorrhagic events have ranged from 12 to 19 in prior reports, with a time lag between bilateral thalamic hemorrhage of up to two days. Herein, we report the first case of delayed (17 days) consecutive contralateral thalamic hemorrhage after spontaneous first thalamic hemorrhage.
A 65-year-old female initially presented with a drowsy mentality with a left-side motor weakness (grade II/III). Brain computed tomography (CT) demonstrated right side thalamic and intraventricular hemorrhage. She regained alertness with mild residual motor weakness (grade III/IV) under medical management. Seventeen days later, a sudden and generalized tonic-clonic seizure developed. Brain CT scans revealed a new contralateral thalamic hemorrhage coincident with microbleeds. Neurologic status remained unchanged, consisting of a stuporous mentality with quadriparesis of grade II/II.
We report the first case of delayed consecutive contralateral thalamic hemorrhage up to 17 days after first thalamic hemorrhage. The case highlights the need for close monitoring of patients with thalamic hemorrhage who experience microbleeds on the contralateral side, due to the possibility of delayed hemorrhage.
Primary multiple intracranial hemorrhage (ICH) without underlying causes of abnormal vascular lesions, trauma or coagulopathy have been reported with an incidence rate ranging from 0.75-4.7% in patients with spontaneous ICH.6)14)17) Putaminal or thalamic hemorrhages are most frequently observed in cases with primary bilateral simultaneous bleedings.20) In previous report, simultaneous or subsequent bilateral thalamic hemorrhages have been reported from 12 up to19 cases.6)7)11) And most of them have been reported in the Asian countries. Bilateral thalamic hemorrhagic events have been reported up to two days apart.11) Here, we report the first case of delayed consecutive contralateral thalamic hemorrhage occurring 17 days after spontaneous thalamic hemorrhage.
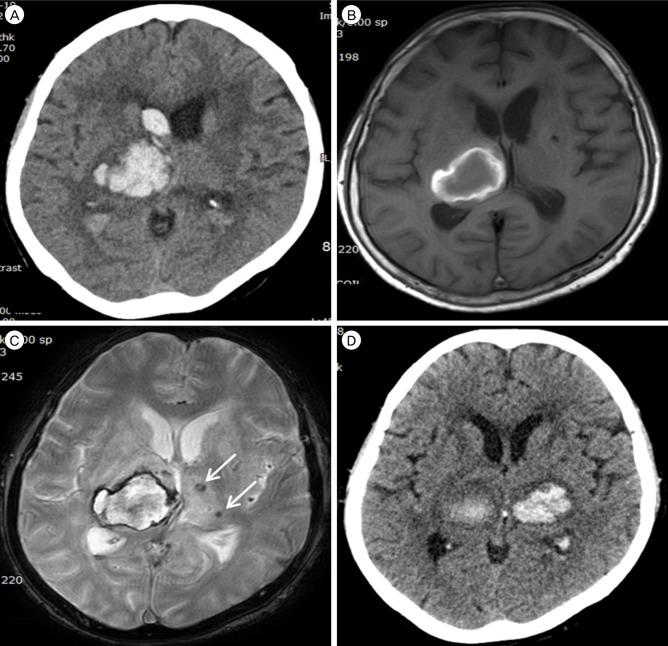
A 65-year-old Korean female presented with drowsy mentality and left side motor weakness (grade II/III). The patient had well-controlled hypertension (HTN) using 5 mg Amlodipine besylate (Novasc®, Korea Pfizer Inc., Seoul, Korea). A laboratory test did not show any signs of coagulopathy. Initial brain computed tomography (CT) scans showed thalamic hemorrhage with intraventricular hemorrhage on the right side (Fig. 1A). Conservative treatments included blood pressure and headache control. Brain magnetic resonance imaging (MRI) 15 days after the first attack revealed subacute thalamic hemorrhage with microblees on the contralateral thalalmus (Fig. 1B, C). Her conscious level gradually improved to alertness with mild residual motor weakness (grade III/IV). Seventeen days later, the patient suddenly experienced generalized tonic-clonic seizure. Brain CT scans showed thalamic hemorrhage on the left side coincident with microbleeds (Fig. 1D). Fosphenytoin (Cerebyx®) was administered for seizure control with a 20 mg/kg loading dose and 6 mg/kg/day maintenance doses. Vertebral artery angiography was done to reveal aneurysms corresponding to thalamic hemorrhage. Aneurysm of the thalamoperforatoring arteries was not observed. The patients remained stuporous with quadriparesis of grade II/III.
Bilateral thalamic hemorrhage can simultaneously or subsequently occur.16)20) However, simultaneous bilateral rupture of perforator arteries or microaneurysm is rare.6) Subsequent rupture could be more appropriate to explain bilateral thalamic hemorrhage than coincidental rupture.6) Second thalamic hemorrhages on the contralateral side have been reported to occur within two days after the first event.11)13) In one case, a patient with herpes simplex encephalitis presented bilateral thalamic hemorrhage with a 4-hour time interval.13) Perez et al.11) reported a case of subsequent hypertensive bilateral thalamic hemorrhage with a two-day time-lag.
The precise mechanism of the bilateral subsequent ICH remains unclear. Systemic diseases such as HTN and atherosclerosis, and old age can contribute to small-vessel diseases (SVD).10) Such pathological conditions include consecutive steps of disorganized tunica media by loss of smooth muscle cells, hyaline deposits and narrowed arterial lumen with thickened wall.5)10) Consequently, rupture of the perforating arteries vulnerable to hemodynamic stress by arterial stiffness or microaneurysm can lead to hemorrhage of deep-seated brain lesions. Presently, brain MRI revealed multiple lacunar infarcts and microbleeds, which represented SVD.8)
Microbleeds are defined as focal blood breakdown products, leakage from the fragile vessel wall.4) Previous studies have shown that microbleeds can be related to hypertensive SVD, old hemorrhage, and ischemic stroke including lacunar infarction.18) Sueda et al.15) reported that hemorrhage sites corresponded to the prior position of microbeeds (odds ratio, 32.3; p < 0.001), in particular deep-seated hemorrhage. Roob et al.12) investigated the microbleeds distribution in ICH patients. Most microbleeds were seen in the cortical-subcortical area (39%) and basal ganglia and thalamic regions (38%). Accordingly, they concluded that microbleeds could be a maker of bleeding prone microangiopathy. The distribution of microbleeds can be helpful to assess the underlying causes of ICH. The predominant location of microbleeds in the cortical-subcortical area, in particular posterior dominance, can suggest cerebral amyloid angiopathy. For hypertensive SVD, microbeeds are more frequently noted in the deep-seated location than cortical-subcortical area. Beyond the pathological changes in intracranial arterial wall, bilateral thalamic hemorrhage also can occur under certain circumstances like venous thrombosis19) or administration of tissue plasminogen activator.3) Neither were relevant in our patient. Therefore, we think that fragility of small perforator arteries to hemodynamic force and the presence of microbleeds could have contributed to the delayed consecutive contralateral thalamic hemorrhage in this patient. Nevertheless, the probability of coexist of hypertensive angiopathy and cerebral amyloid angiopathy cannot be fully excluded.
Another possible mechanism can be a change in autoregulation after hemorrhage development. Nakagawa et al.9) reported that patients with ICH have higher middle cerebral artery transfer gains with a wide range of frequency on both sides as compared to healthy controls. This result implies that patients with ICH have less effective cerebral autoregulation than healthy individuals. Accordingly, initial hemorrhage insult could increase blood pressure, which might result in contralateral hemorrhage due to less effective autoregulation.6)
Some physicians may regard our case report as a recurrent hypertensive hemorrhage due to relative long-term interval of 17 days between the two hemorrhagic events. However, previous studies have shown recurrent hemorrhage events at least 1-month from initial hemorrhage insult. Chen et al.2) reported 47 cases of recurrent hypertensive hemorrhage with the median interval of 2.3 years (range: 1 month to 8.5 years). Bae et al.1) reported a 5.4% rate of recurrent hypertensive hemorrhage with the median interval of 22.3 months (range: 1.7-71.9 months). Accordingly, we suggest that our case could be more appropriate to describe delayed consecutive contralateral thalamic hemorrhage than recurrent thalamic hemorrhage.
Delayed consecutive contralateral thalamic hemorrhage up to 17 days can occur after first thalamic hemorrhage. Due to the poor clinical outcome of bilateral thalamic hemorrhage, patients with thalamic hemorrhage who experienced microbleeds should be monitored closely due to the possibility of delayed contralateral thalamic hemorrhage.
References
1. Bae H, Jeong D, Doh J, Lee K, Yun I, Byun B. Recurrence of bleeding in patients with hypertensive intracerebral hemorrhage. Cerebrovasc Dis. 1999; Mar-Apr. 9(2):102–108. PMID: 9973653.

2. Chen ST, Chiang CY, Hsu CY, Lee TH, Tang LM. Recurrent hypertensive intracerebral hemorrhage. Acta Neurol Scand. 1995; 2. 91(2):128–132. PMID: 7785422.

3. Dromerick AW, Meschia JF, Kumar A, Hanlon RE. Simultaneous bilateral thalamic hemorrhages following the administration of intravenous tissue plasminogen activator. Arch Phys Med Rehabil. 1997; 1. 78(1):92–94. PMID: 9014966.

4. Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, Schmidt R, et al. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol. 1999; 4. 20(4):637–642. PMID: 10319975.
5. Furuta A, Ishii N, Nishihara Y, Horie A. Medullary arteries in aging and dementia. Stroke. 1991; 4. 22(4):442–446. PMID: 2024272.

6. Kono K, Terada T. Simultaneous bilateral hypertensive putaminal or thalamic hemorrhage: case report and systematic review of the literature. Turk Neurosurg. 2014; 24(3):434–437. PMID: 24848190.

7. Laiwattana D, Sangsawang B, Sangsawang N. Primary multiple simultaneous intracerebral hemorrhages between 1950 and 2013: analysis of data on age, sex and outcome. Cerebrovasc Dis Extra. 2014; 5. 4(2):102–114. PMID: 24932180.

8. Martinez-Ramirez S, Greenberg SM, Viswanathan A. Cerebral microbleeds: overview and implications in cognitive impairment. Alzheimers Res Ther. 2014; 6. 6(3):33. PMID: 24987468.

9. Nakagawa K, Serrador JM, LaRose SL, Sorond FA. Dynamic cerebral autoregulation after intracerebral hemorrhage: A case-control study. BMC Neurol. 011; 8. 11:108. PMID: 21884574.

10. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010; 7. 9(7):689–701. PMID: 20610345.

11. Perez J, Scherle C, Machado C. Subsequent bilateral thalamic haemorrhage. BMJ Case Rep. 2009; 2009.

12. Roob G, Lechner A, Schmidt R, Flooh E, Hartung HP, Fazekas F. Frequency and location of microbleeds in patients with primary intracerebral hemorrhage. Stroke. 2000; 11. 31(11):2665–2669. PMID: 11062292.

13. Sakaguchi J, Yonemura K, Hashimoto Y, Hirano T, Uchino M. Herpes simplex encephalitis originating from bilateral thalamic lesions with hemorrhagic component. Rinsho Shinkeigaku. 2005; 5. 45(5):368–371. PMID: 15960174.
14. Stemer A, Ouyang B, Lee VH, Prabhakaran S. Prevalence and risk factors for multiple simultaneous intracerebral hemorrhages. Cerebrovasc Dis. 2010; 8. 30(3):302–307. PMID: 20664265.

15. Sueda Y, Naka H, Ohtsuki T, Kono T, Aoki S, Ohshita T, et al. Positional relationship between recurrent intracerebral hemorrhage/lacunar infarction and previously detected microbleeds. AJNR Am J Neuroradiol. 2010; 9. 31(8):1498–1503. PMID: 20448017.

16. Sunada I, Nakabayashi H, Matsusaka Y, Nishimura K, Yamamoto S. Simultaneous bilateral thalamic hemorrhage: case report. Radiat Med. 1999; Sep-Oct. 17(5):359–361. PMID: 10593286.
17. Tanno H, Ono J, Suda S, Karasudani H, Yamakami I, Isobe K, et al. Simultaneous, multiple hypertensive intracerebral hematomas: report of 5 cases and review of literature. No Shinkei Geka. 1989; 3. 17(3):223–228. PMID: 2671770.
18. Viswanathan A, Chabriat H. Cerebral microhemorrhage. Stroke. 2006; 2. 37(2):550–555. PMID: 16397165.

19. Wang PY, Shen WC. MRI in internal cerebral vein thrombosis: case note. Neuroradiology. 1995; 8. 37(6):447–448. PMID: 7477853.

20. Yen CP, Lin CL, Kwan AL, Lieu AS, Hwang SL, Lin CN, et al. Simultaneous multiple hypertensive intracerebral haemorrhages. Acta Neurochir (Wien). 005; 4. 147(4):393–399. PMID: 15605198.

Fig. 1
(A) Brain computed tomography (CT) scans shows right-side thalamic hemorrhage with intraventricular hemorrhage. (B, C) Magnetic resonance imaging 15 days after initial thalamic hemorrhage shows subacute stage hematoma with microbleeds (arrows) on the contralateral thalalmus. (D) Emergent CT 17 days after first thalamic hemorrhage reveals a new contralateral thalamic hemorrhage coincident with microbleeds.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download