Abstract
A basilar artery dissection (BAD) is an extremely rare disease. It can lead to hemorrhage or infarction involving the brain stem, and is often associated with grave outcome. However, little is known about the pathophysiology of BAD, and its proper managements are yet in controversy. Herein, we report on two rare cases of basilar artery dissection from strenuous physical effort; one from sexual intercourse and another from defecation. The treatment modalities and the outcomes are also discussed.
A basilar artery dissection (BAD) is an extremely rare disease and often associated with grave outcomes.5)11)12)15)22) Expansion of the mural hematoma causes luminal stenosis, and eventually brings hemodynamic insufficiencies distal to the basilar artery and at the pontine perforating arteries of the diseased segment. On the other hand, enlargement of the false lumen causes fusiform aneurysmal dilatation, and the fragile portion can be ruptured. Although the etiology of BAD remains to be unclear in many cases, factors such as atherosclerosis, cervical trauma, cervical manipulation, and fibromuscular dysplasia are generally thought to be related with the occurrence of BAD.4)5)11)12)14)
Since a dissection confined to the basilar artery has much lower incidence than a vertebral artery dissection and the prognosis is inconsistent, the proper managements for BAD are often in controversy.1)13) Aggressive manipulations of the diseased segment or rough handlings of instruments may lead to a devastating result such as an arterial rupture due to the instability of the intimal flap and the fragility of the false lumen.
Herein, we report on two rare cases of BAD from strenuous physical effort; one from sexual intercourse and another from defecation. The treatment modalities and the outcomes are also discussed.
A 28-year-old male without any previous medical history experienced right hemiplegia after a paroxysmal headache during sexual intercourse. He had a drowsy mentality and dysarthria. The National Institutes of Health Stroke Scale (NIHSS) score was 12. The brain magnetic resonance image (MRI) showed a pontine infarction (Fig. 1). The anterior-posterior view of his vertebral angiogram demonstrated an abrupt filling defect at the mid-basilar artery where the proximal margin of the defect was somewhat blurred and the distal was clearly circumscribed. On the lateral view, the abrupt filling defect was located at the anterior wall of the artery and the distal flow was compromised by a luminal stenosis (Fig. 2A, B). These angiographic findings were highly suggestive of an anterior wall dissection of the basilar artery where the false lumen was filled up with mural hematoma.
Since his neurological status had been deteriorating, a subsequent endovascular procedure was performed. After confirmation of the anatomical relationship between the true and false lumens, a microwire was navigated into the distal portion across the true lumen with extremely delicate handling to avoid incorrect cannulation into the false lumen. At the first trial of microwire navigation, it went into the intramural hematoma, and a clear resistance was noticed. Then, it was slightly pulled back and advanced again following the posterior wall of the diseased segment for a successful navigation. This maneuver confirmed presence of BAD. Then, a stent was deployed fully covering the lesion including some safety margins. After the stenting procedure, the distal flow was completely restored without residual false lumen (Fig. 2C, D). A week later, he was able to walk unassisted. He discharged without any neurological deficits.
An 80-year-old male with severe constipation experienced a sudden headache during defecation. Shortly after, he visited local clinic for medication and abrupt left hemiplegia occurred. He was in a drowsy state and had dysarthria. The NIHSS score was 12. The brain MRI revealed a pontine infarction (Fig. 3A). The T1-weighted image demonstrated a mural thickening with hyperintensity at the right anterior wall of the basilar artery (Fig. 3B). On the vertebral angiogram, a contrast filling defect was found on the right anterior wall of the mid-basilar artery which was responsible for the luminal stenosis. Based on these radiological findings, a right anterior wall dissection of the basilar artery with mural hematoma was suspected. Similar to those angiographic findings on Case 1, the postero-superior border of the defect where the intimalflap might exist had a smooth and clear margin, and the inferior border where the mural hematoma and false lumen might be exposed had an irregular and unclear margin (Fig. 4A, B).
During the angiographic evaluation, his motor power dramatically improved to grade III. He had full recovery after conservative treatments including administration of low-molecular heparin and antiplatelet agent. Follow-up vertebral angiogram after two weeks demonstrated a fusiform aneurysmal dilatation with clearly visible intimal flap (Fig. 5A). Since such aneurysmal formation caused by the enlargement of the false lumen has an extremely fragile wall and its rupture seemed to have a high mortality rate, an endovascular treatment was performed. Double stents were inserted into the true lumen fully covering the diseased segment. After the stenting, the false lumen was somewhat collapsed, but some residual false lumen was still visible (Fig. 5B). Follow-up angiogram at two months after the initial ischemic onset demonstrated a complete healing of the dissection (Fig. 5C). He remained in a symptom free state.
Dissection of intracranial arterial system is rare. Approximately 90% of these dissections occur in the posterior circulations, and most of the previous reports focus on the dissections of the vertebral artery.7)8)19)21)22) The incidence of vertebral artery dissection is approximately 1-1.5 per 100,000,2) and its mortality rate is estimated to be 17-46%.10) However, dissections confined to the basilar artery have been reported much less frequently and little is known about the clinical course, proper managements, and prognosis. Although extremely rare, a BAD generally seemed to have even a worse prognosis than a vertebral artery dissection. The cause of BAD remains unclear but it can be associated with atherosclerosis, cervical trauma, cervical manipulation, and fibromuscular dysplasia.4)5)12)13)15) Strenuous physical efforts such as sexual intercourse and defecation, as in our report, can also be causative factors.3) However, it is not yet proven how these physical stresses can mechanically induce strain in the vessel and initiate the arterial dissection. Despite rare incidence and obscure cause of BAD, the outcome can be tragic because BAD can lead to hemorrhage or infarction of the brain stem.6)9) Hence, strict neurological examinations and appropriate image work-ups are significant to prevent a misdiagnosis.
MRI is a useful tool for diagnosis of BAD because it is non-invasive and may demonstrate the presence of intramural hematoma.11) Nonetheless, conventional angiography is a crucial diagnostic tool for confirmation of the hemodynamic status and, moreover, the anatomical configuration including the relationship between the true and false lumens.16) In addition to the result of image work-ups, the severity of neurological status is important factor for deciding the treatment strategy. Moreover, regular image follow-ups such as MRI and conventional angiography are crucial especially for the patients who underwent conservative treatments because BAD can have a dynamic clinical course with changes in anatomical configuration.
There have been controversies on the modality of treatment for BAD. Pozzati et al. advocated conservative treatment because of possibility of spontaneous healing of dissection and risk of the surgical interventions.17) Willing et al. referred to risk of endovascular intervention for BAD, which can be evoked if a false lumen is incorrectly cannulated.20) However, some other authors have emphasized the necessity and efficacy of stenting and other procedures.9)18) Shin et al.18) mentioned that although stenting for BAD may have some technical difficulties and carry procedural risks, it can be the only effective method for management of acute symptomatic BAD if incorrect cannulation into a false lumen is avoided.
Our cases represent that a strenuous physical effort even without underlying vasculopathy as in Case 1 or direct trauma as in Case 1 and 2 can possibly be a causative factor for occurrence of BAD. In the Case 1, the patient suffered from progressive brain stem ischemia with profound hemodynamic insufficiency, and an immediate endovascular treatment led to dramatic improvements of the symptoms. In this case, stenting would not be beneficial for already sacrificed basilar perforators which caused the pontine infarction, but would be beneficial for partially compromised perforators and those supplied by insufficient perfusion pressure mostly at the distal to the lesion. Moreover, stenting not only improved perfusion but also prevented progression of the dissection by inhibiting enlargement of the false lumen. In the Case 2, the patient showed some improvements at the early stage and underwent a conservative treatment. However, due to formation of a fusiform aneurysmal dilatation, he underwent stenting in order to prevent a rupture. Stenting might stabilize the intimal flap and enhance healing of dissection, thereby reducing the period exposed to the risk of hemorrhage. In the both cases, judicious approach and delicate handling of instruments were required for successful navigation across the true lumen, and their outcomes after endovascular treatments were excellent. Based on our experiences and previous reports, stenting is effective and technically feasible for the treatment of BAD if a correct cannulation into the true lumen is possible. We suggest that endovascular treatments such as stenting should be cautiously recommended for hemorrhagic or ruptured BAD, recurrent or progressive hemodynamic ischemia due to BAD, and relatively large or growing aneurysmal formation at the false lumen. We also emphasize the necessity of follow-up angiography for those who underwent conservative treatments at the early stage, and if subsequent aneurysmal formation is evident, an endovascular therapy is required in order to prevent a hemorrhagic crisis.
Although BAD is extremely rare, it is potentially a life-threatening disease. Strenuous physical effort such as sexual intercourse or defecation can be one of causative factors. Detailed inspection of MRI and cerebral angiography is essential for the accurate diagnosis and proper treatment. Stenting can be a feasible treatment option for management of BAD. Precise navigation into the true lumen with delicate control of instruments is the key to the successful and safe endovascular treatment.
References
1. Berkovic SF, Spokes RL, Anderson RM, Bladin PF. Basilar artery dissection. J Neurol Neurosurg Psychiatry. 1983; 2. 46(2):126–129. PMID: 6842215.

2. Bogousslavsky J, Regli F. Ischemic stroke in adults younger than 30 years of age: cause and prognosis. Arch Neurol. 1987; 5. 44(5):479–482. PMID: 3579657.
3. Delasobera BE, Osborn SR, Davis JE. a case of basilar artery dissection associated with sexual intercourse. J Emerg Med. 2012; 7. 43(1):e43–e47. PMID: 19818575.
4. Endo S, Nishijima M, Nomura H, Takaku A, Okada E. A pathological study of intracranial posterior circulation dissecting aneuryms with subarachnoid hemorrhage: report of three autopsied cases and review of the literature. Neurosurgery. 1993; 10. 33(4):732–738. PMID: 8232816.
5. Han Z, Leung TW, Lam W, Soo Y, Wong K. Spontaneous basilar artery dissection. Hong Kong Med J. 2007; 4. 13(2):144–146. PMID: 17406043.
6. Higashida RT, Smith W, Gress D, Urwin R, Dowd CF, Balousek PA, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery. J Neurosurg. 1997; 12. 87(6):944–949. PMID: 9384409.

7. Im TS, Lee YS, Suh SJ, Lee JH, Ryu KY, Kang DG. Two cases of subarachnoid hemorrhage from spontaneous aneterior cerebral artery dissection: a case of simultaneous hemorrhage and ischemia without aneurismal formation and another case of hemorrhage with aneurysmal formation. J Cerebrovasc Endovasc Neurosurg. 2014; 6. 16(2):119–124. PMID: 25045652.
8. Inoue T, Fujimura M, Matsumoto Y, Kondo R, Inoue T, Shimizu H, et al. Simultaneous occurrence of subarachnoid hemorrhage and cerebral infarction caused by anterior hemorrhage and cerebral infarction caused by anterior cerebral artery dissection treated by endovascular trapping. Neurol Med Chir (Tokyo). 010; 7. 50(7):574–577. PMID: 20671384.
9. Kim BM, Suh SH, Park SI, Shin YS, Chung EC, Lee MH, et al. Management and Clinical Outcome of Acute Basilar Artery Dissection. AJNR Am J Neuroradiol. 2008; 11. 29(10):1937–1941. PMID: 18687744.

10. Kim CH, Son YJ, Paek SH, Han MH, Kim JE, Chung YS, et al. Clinical analysis of vertebrobasilar dissection. Acta Neurochir (Wien). 006; 4. 148(4):395–404. PMID: 16511630.

11. Kitanaka C, Tanaka J-I, Kuwahara M, Teraoka A. Magnetic resonance imaging study of intracranial vertebrobasilar artery dissections. Stroke. 1994; 3. 25(3):571–575. PMID: 8128509.

12. Lee MS, Lee YS, Lee JH, Ryu KY, Kang DG. A case of actue basilar artery dissection associated with sexual intercourse: a life-trheatening cause of coital cephalgia. J Korean Soc Intravasc Neurosurg. 2011; 6. 6(1):42–46.
13. Malek AM, Halbach VV, Phatouros CC, Meyers PM, Dowd CF, Higashida RT. Endovascular treatment of a ruptured intracranial dissecting vertebral aneurysm in a kickboxer. J Trauma. 2000; 1. 48(1):143–145. PMID: 10647582.

14. Manz HJ, Vester J, Lavenstein B. Dissecting aneurysm of cerebral arteries in childhood and adolescence. case report and literature review of 20 cases. Virchows Arch A Pathol Anat Histol. 1979; 10. 384(3):325–335. PMID: 160123.
15. Nakahara T, Satoh H, Mizoue T, Kawamoto H, Kohmo Y, Kurisu K. Dissecting aneurysm of basilar artery presenting with recurrent subarachnoid hemorrhage. Neurosurg Rev. 1999; 10. 22(2-3):155–158. PMID: 10547021.

16. Nakatomi H, Nagata K, Kawamoto S, Furusho JI. Basilar artery occlusion due to spontaneous basilar artery dissection in a child. Acta Neurochir (Wien). 999; 1. 141(1):99–104. PMID: 10071693.

17. Pozaati E, Andreoli A, Padovani R, Nuzzo G. Dissecting aneurysms of the basilar artery. Neurosurgery. 1995; 2. 36(2):254–258. PMID: 7731504.

18. Shin YS, Kim HS, Kim SY. Stenting for vertebrobasilar dissection: a possible treatment option for nonhemorrhagic vertebrobasilar dissection. Neuroradiology. 2006; 2. 49(2):149–156. PMID: 17131115.

19. Suzuki K, Mishina M, Okubo S, Abe A, Suda S, Ueda M, et al. Anterior cerebral artery dissection presenting subarachnoid hemorrhage and cerebral infarction. J Nippon Med Sch. 2012; 4. 79(2):153–158. PMID: 22687360.

20. Willing SJ, Skidmore F, Donaldson J, Nobo UL, Chernukha K. Treatment of acute intracranial vertebrobasilar dissection with angioplasty and stent placement: report of two cases. AJNR Am J Neuroradiol. 2003; 5. 24(5):985–989. PMID: 12748108.
21. Yamaura A, Ono J, Hirai S. Clinical picture of intracranial non-traumatic dissecting aneurysm. Neuropathology. 2000; 3. 20(1):85–90. PMID: 10935444.

22. Yasukawa K, Kamijo Y, Momose G, Kobayashi S, Ikeda A. A case of anterior cerebral artery dissecting aneurysm presenting subarachnoid hemorrhage and cerebral infarction at the same time. Surg Cereb Stroke. 1993; 10. 21(6):461–466.

23. Yu J, Xu K, Wang H, Wang B, Luo Q. Endovascular coil embolization of parent artery for giant intracranial basilar artery dissection: a case report. Turk Neurosurg. 2012; 7. 22(4):483–488. PMID: 22843471.

Fig. 2
Antero-posterior (AP) view (A) and lateral view (B) of vertebral angiogram demonstrate an abrupt filling defect at the anterior wall of the mid-basilar artery. AP (C) and lateral (D) views after stenting show completely restored distal flow.

Fig. 3
Diffusion-weighted MRI reveals a pontine infarction (A). The hyperintense lesion (white arrow) at the right anterior wall of the basilar artery on T1-weighted MRI indicates mural hematoma (B). MRI = magnetic resonance imaging.
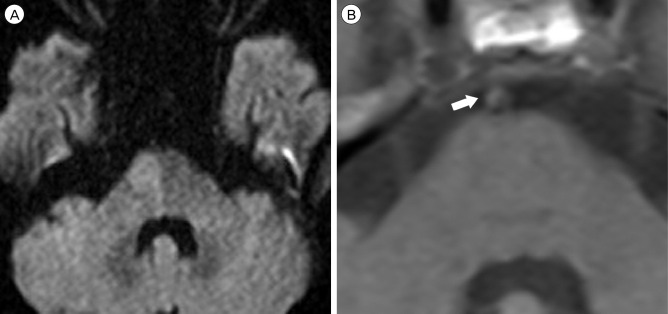
Fig. 4
AP view (A) and left anterior oblique view (B) of vertebral angiogram show a contrast filling defect (white arrows) at the right anterior wall of the mid-basilar artery. AP = antero-posterior.
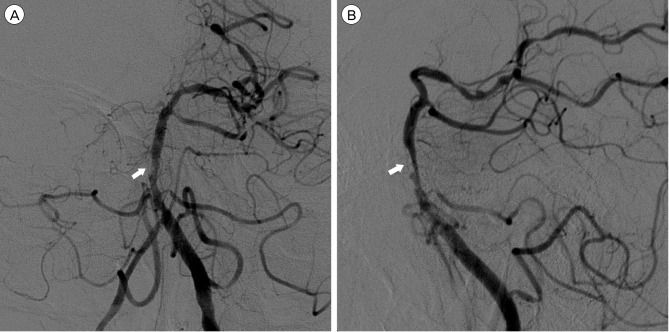
Fig. 5
Left anterior oblique view (A) of follow-up vertebral angiogram after two weeks shows a fusiform aneurysmal dilatation of the false lumen. Note that the intimal flap is clearly visualized. Immediate post-procedural angiogram (B) reveals some residual false lumen although it is somewhat collapsed. Follow-up angiogram (C) in a similar projection angle at two months after the initial ischemic onset shows a complete healing of the dissection.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download