Abstract
Reversible cerebral vasoconstriction syndrome (RCVS) is a group of syndromes characterized by reversible segmental constriction of cerebral arteries. Posterior reversible encephalopathy syndrome (PRES) is another clinical-radiologic syndrome characterized by reversible, posterior-predominant brain edema. Although the exact causes of these reversible syndromes are poorly understood, these entities may share some common pathophysiologic elements leading to hemorrhagic strokes and rarely, deep intracerebral hemorrhage (ICH). Recent studies have suggested that endothelial dysfunction is a common pathophysiologic factor associated with these syndromes. We report on two young female patients who presented with deep ICH and were later diagnosed as RCVS and PRES. Both patients suffered from vasoconstriction and delayed ischemic stroke. Early detection of distinguishing clinical-radiologic features associated with these reversible syndromes and removing triggers would facilitate successful treatment with no complications.
Reversible cerebral vasoconstriction syndrome (RCVS) is a group of syndromes characterized by reversible segmental constriction of cerebral arteries, typically associated with severe headaches and often complicated by ischemic or hemorrhagic stroke.11) One-third of patients initially present with hemorrhagic stroke, typically isolated cortical subarachnoid hemorrhage with or without superficial intracerebral hemorrhage (ICH). Some patients (less than 3%) may present with isolated deep ICH, making differential diagnosis challenging.13) Posterior reversible encephalopathy syndrome (PRES) is an another clinical-radiologic syndrome characterized by reversible, posterior-predominant brain edema, usually associated with headache, altered mental status, seizures, and visual symptoms, but also often complicated by stroke.14)17) In a retrospective study of 263 PRES patients, 46 patients (17%) presented with ICH but only 4 (1.5%) had deep ICH.18) Although the exact causes of these reversible syndromes are poorly understood, these entities may share some common pathophysiologic elements leading to hemorrhagic stroke and, rarely, deep ICH.11) We report on two young female patients who presented with deep ICH and were later diagnosed as RCVS and PRES.
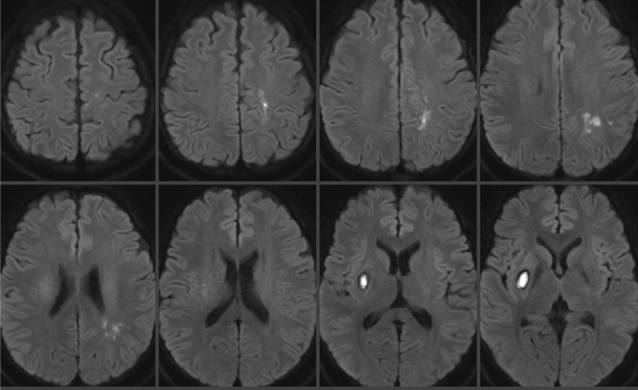
A 29-year-old female patient with a 6-day history of laparoscopic uterine myomectomy visited a local hospital complaining of worsening headache and mild left hand weakness since surgery. Brain computed tomography (CT) showed acute ICH in the right basal ganglia and no enhancing lesion within the hemorrhage was observed on CT angiography (CTA). However, mild multifocal stenoses were detected on the bilateral distal middle cerebral arteries (MCA), which had gone undetected at that time (Fig. 1). She had developed severe anemia (5.3 g/dL) attributable to menorrhagia and required a red blood cell transfusion before and after myomectomy. In addition, because of uncontrolled vaginal bleeding after myomectomy, uterine constrictors (oxytocin and methylergometrine) were administered 2 days after myomectomy. Right basal ganglia ICH was managed conservatively, but she developed right side weakness three days after ICH diagnosis. Diffusion weighted magnetic resonance imaging (DWI) performed at that time showed multifocal border zone infarctions in the left hemisphere (Fig. 2). She was transferred to our institute for evaluation of concomitant hemorrhagic and ischemic strokes 14 days after myomectomy (8 days after ICH diagnosis and 5 days after infarction diagnosis).
Brain MR angiography (MRA) performed upon arrival showed multifocal stenoses involving the bilateral anterior cerebral arteries (ACA), MCA, posterior cerebral arteries (PCA), superior cerebellar arteries, and basilar artery without wall enhancement of affected arteries on high resolution MR wall imaging (Fig. 3). With a presumptive diagnosis of RCVS, the patient was managed with nimodipine. Transfemoral cerebral angiography performed 6 days later showed improving but residual multifocal stenoses (Fig. 4). The patient was discharged with no significant disability and 6-month follow-up brain magnetic resonance imaging (MRI) and MRA confirmed complete resolution of multifocal stenoses with no new parenchymal lesion (Fig. 5).
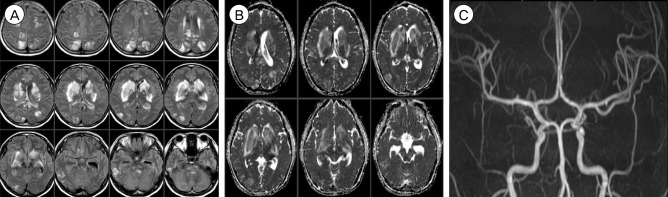
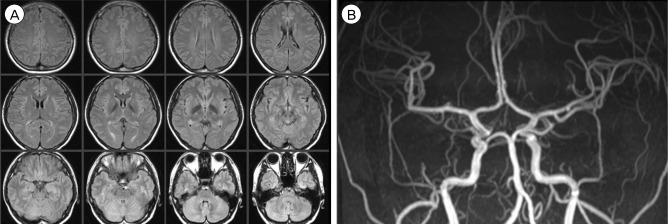
A 31-year-old female patient underwent emergent Cesarean section for a 36-week twin pregnancy under spinal anesthesia. Immediately after uneventful surgery, she complained of surgical wound pain and her systolic blood pressure rose to 160 mmHg (mean arterial pressure 128 mmHg). An opioid analgesic (morphine) was administered, but she became unconscious and developed a generalized seizure 5 hours later. Brain CT showed acute ICH in the head of the right caudate nucleus and associated intraventricular hemorrhage (IVH). In addition, symmetrically scattered low density lesions were observed in the bilateral basal ganglia and parietal lobes (Fig. 6A). CTA did not show any enhancing lesion within the hemorrhage or any abnormality in the major cerebral arteries as well as the major cerebral veins and sinuses (Fig. 6B). Laboratory findings showed proteinuria, hemolytic anemia, elevated liver enzymes (aspartate aminotransferase, 312 IU/L; alanine aminotransferase, 154 IU/L), and low platelet count (60 × 103/µL), which were suggestive of eclampsia (seizure, hypertension, proteinuria) and HELLP (Hemolysis, Elevated liver enzymes, Low platelet count) syndrome. Subsequent brain MRI taken a few hours later confirmed that the low density lesions observed on CT were composed of larger areas of increased apparent diffusion coefficient values, suggesting vasogenic edema, and smaller areas of diffusion restriction and enhancement. These edematous lesions involved not only the bilateral basal ganglia and parietal lobes but also the bilateral frontal lobes, cingulate gyrus, corpus callosum, midbrain, and pons. MRA was normal (Fig. 7).
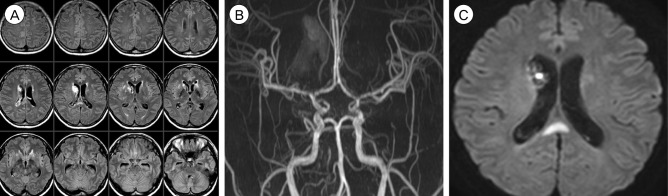
She was diagnosed as PRES associated with eclampsia, based on the characteristic imaging and laboratory findings. In addition to platelet concentrate transfusion, the patient was managed with antihypertensive (labetalol), antiepileptic (levetiracetam), antifibrinolytic (tranexamic acid), and hyperosmolar (mannitol) drugs. Two days later, she regained her consciousness with no fixed neurological deficits. Laboratory findings were nearly normalized and follow-up brain CT showed decreased edema and IVH without evidence of hydrocephalus. However, 5 days later, she complained of severe headache, nausea, and vomiting. Follow-up brain MRI showed further decrement of bilateral edema but MRA showed newly appeared multifocal stenoses involving the bilateral MCA, ACA, and distal internal carotid arteries and an associated delayed ischemic lesion on the splenium of the corpus callosum (Fig. 8). Multifocal stenoses showed gradual improvement on transcranial Doppler monitoring with the use of nimodipine and the patient was discharged with no neurological deficit 14 days after Cesarean section. Follow-up brain MRI and MRA taken 3 months later showed complete resolution of vasogenic edema and multifocal stenoses (Fig. 9).
The mechanisms involved in RCVS remain uncertain. However, a disturbance in the control of cerebral vascular tone appears to be a critical element and this alteration in vascular tone may be spontaneous or evoked by various endogenous or exogenous factors.5) The use of vasoactive substances, such as sympathomimetics, serotonin-reuptake inhibitors, and ergoline derivatives, is the common precipitating factor of RCVS.12) In our case 1, the use of an ergoline uterine constrictor (methylergometrine) could have been the trigger of RCVS.12)13) Also, it has been reported that transfusion can be a potential trigger of RCVS.10) The patient initially had basal ganglia ICH but later experienced multifocal border zone infarctions attributable to RCVS progression. However, early diagnosis of RCVS is sometimes difficult because the initial vascular imaging may be normal or demonstrate only mild narrowing of distal small arteries.6)11)12)16) Repeated vascular imaging after a few days or a week would demonstrate the progression of multifocal vasoconstriction toward proximal cerebral arteries as shown in our case 1.6)11)13) Thus, a high index of suspicion is required and repeated vascular imaging should be considered in young women with severe headache and cryptogenic deep ICH.
The diagnosis of PRES is based on relatively symmetric vasogenic edema of the white matter predominately localized in the area of posterior circulation, particularly in the parieto-occipital regions. Other structures including the brain stem, cerebellum, basal ganglia, and frontal lobes can occasionally be involved.15) Our case 2 was initially diagnosed as PRES considering the symmetrically scattered lesions, especially in the parietal lobes. However, the patient later developed multifocal vasoconstriction and delayed infarction. Multifocal vasoconstriction has been reported in more than 85% of PRES patients and this vasoconstriction was shown to be reversible on follow-up imaging studies.11) The mechanisms of PRES are also not well understood. The most plausible theory for PRES is that acute hypertension causes a breakdown of blood-brain barrier and resultant vasogenic edema.2) The predilection for the posterior circulation area would be explained by sparse sympathetic innervation of the vertebrobasilar system.1)19) However, acute hypertension is absent in 25% to 29% of PRES patients.1)14) Even in patients with acute hypertension, mean arterial pressure exceeding the autoregulatory upper limit of 150mmHg is uncommon, as observed in our case 2.1)2) PRES can develop in patients with complex systemic conditions such as eclampsia, sepsis, autoimmune disease, after cancer chemotherapy, and after transplantation.1) In our case 2, PRES occurred in association with hypertension and eclampsia. Recent studies have suggested an association of eclampsia and preeclampsia with endothelial dysfunction and resultant hypertension.2)4) Increased permeability of the blood-brain barrier, presumed to be the key pathogenic mechanism of PRES, might be attributable to endothelial dysfunction in various clinical conditions and diseases.17)
RCVS and PRES share many clinical-radiologic features and treatment modalities as described here. Both in RCVS and PRES, the clinical and neuro-imaging findings tend to be reversible once the underlying cause of these syndromes is identified and treated.1)2)5) Imaging findings compatible with PRES were observed in 9 to 38% of RCVS patients.11) A recent MRA study reported that more severe vasoconstriction in the M1 and P2 segments contributed to a higher risk of PRES in RCVS patients.7) Also, PRES patients may develop reversible vasoconstriction of cerebral vessels.3) Endothelial dysfunction is thought to be an important pathogenic factor in PRES2)17) and some studies have suggested an association between RCVS and endothelial dysfunction.8)9) Endothelial dysfunction can lead to failure of cerebral autoregulation, and it may precipitate the rupture of small cerebral vessels with resulting hemorrhagic strokes including deep ICH.13)14) A spectrum of vascular dysregulation resultant by endothelial dysfunction in various systemic conditions may be a key pathophysiologic factor of these syndromes.
We report on two young women who presented with deep ICH and were later diagnosed as RCVS and PRES. Both patients suffered from vasoconstriction and delayed ischemic strokes. Early detection of distinguishing clinical-radiological features associated with these reversible syndromes and removing triggers would facilitate successful treatment with no complications.
References
1. Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008; 6. 29(6):1036–1042. PMID: 18356474.

2. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008; 6. 29(6):1043–1049. PMID: 18403560.

3. Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2008; 3. 29(3):447–455. PMID: 18079186.

4. Brandes RP. Endothelial dysfunction and hypertension. Hypertension. 2014; 11. 64(5):924–928. PMID: 25156167.

5. Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007; 1. 146(1):34–44. PMID: 17200220.

6. Call GK, Fleming MC, Sealfon S, Levine H, Kistler JP, Fisher CM. Reversible cerebral segmental vasoconstriction. Stroke. 1988; 9. 19(9):1159–1170. PMID: 3046073.

7. Chen SP, Fuh JL, Wang SJ, Chang FC, Lirng JF, Fang YC, et al. Magnetic resonance angiography in reversible cerebral vasoconstriction syndromes. Ann Neurol. 2010; 5. 67(5):648–656. PMID: 20437562.

8. Chen SP, Fuh JL, Wang SJ, Tsai SJ, Hong CJ, Yang AC. Brain-derived neurotrophic factor gene Val66Met polymorphism modulates reversible cerebral vasoconstriction syndromes. PLoS One. 2011; 3. 6(3):e18024. PMID: 21437208.

9. Chen SP, Wang YF, Huang PH, Chi CW, Fuh JL, Wang SJ. Reduced circulating endothelial progenitor cells in reversible cerebral vasoconstriction syndrome. J Headache Pain. 2014; 12. 15:82. PMID: 25466718.

10. Dou YH, Fuh JL, Chen SP, Wang SJ. Reversible cerebral vasoconstriction syndrome after blood transfusion. Headache. 2014; 4. 54(4):736–744. PMID: 24628283.

11. Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol. 2012; 10. 11(10):906–917. PMID: 22995694.

12. Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007; 12. 130(Pt 12):3091–3101. PMID: 18025032.

13. Ducros A, Fiedler U, Porcher R, Boukobza M, Stapf C, Bousser MG. Hemorrhagic manifestations of reversible cerebral vasoconstriction syndrome: frequency, features, and risk factors. Stroke. 2010; 11. 41(11):2505–2511. PMID: 20884871.
14. Hefzy HM, Bartynski WS, Boardman JF, Lacomis D. Hemorrhage in posterior reversible encephalopathy syndrome (PRES): Imaging and clinical features. AJNR Am J Neuroradiol. 2009; 8. 30(7):1371–1379. PMID: 19386731.
15. Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996; 2. 334(8):494–500. PMID: 8559202.

16. Marder CP, Donohue MM, Weinstein JR, Fink KR. Multimodal imaging of reversible cerebral vasoconstriction syndrome: a series of 6 cases. AJNR Am J Neuroradiol. 2012; 8. 33(7):1403–1410. PMID: 22422190.

17. Marra A, Vargas M, Striano P, Del Guercio L, Buonanno P, Servillo G. Posterior reversible encephalopathy syndrome: the endothelial hypotheses. Med Hypotheses. 2014; 5. 82(5):619–622. PMID: 24613735.

18. Sharma A, Whitesell RT, Moran KJ. Imaging pattern of intracranial hemorrhage in the setting of posterior reversible encephalopathy syndrome. Neuroradiology. 2010; 10. 52(10):855–863. PMID: 19956935.

19. Sheth RD, Riggs JE, Bodenstenier JB, Gutierrez AR, Ketonen LM, Ortiz OA. Parietal occipital edema in hypertensive encephalopathy: a pathogenic mechanism. Eur Neurol. 1996; 1. 36(1):25–28. PMID: 8719646.

Fig. 1
(A) Brain CT shows the isolated deep ICH in the right basal ganglia. (B) Brain CTA shows multifocal narrowing of distal MCA branches (arrows). CT = computed tomography; ICH = intracerebral hemorrhage; CTA = CT angiography; MCA = middle cerebral arteries.
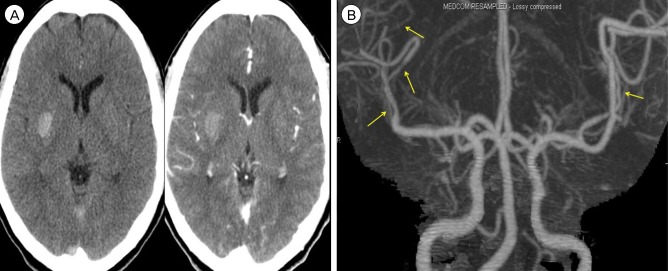
Fig. 3
(A) MRA shows the progression of multifocal vasoconstriction toward proximal cerebral arteries. (B) There is no wall enhancement of affected vessels on high resolution MR wall imaging. MRA = MR angiography; MR = magnetic resonance.
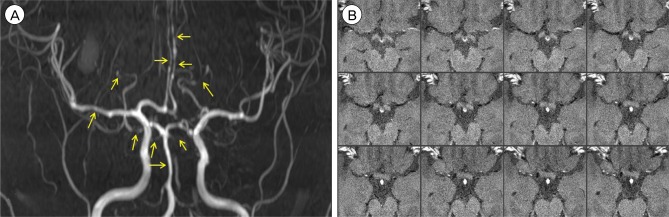
Fig. 4
Digital subtraction angiography demonstrates improving but residual vasoconstriction (arrows).
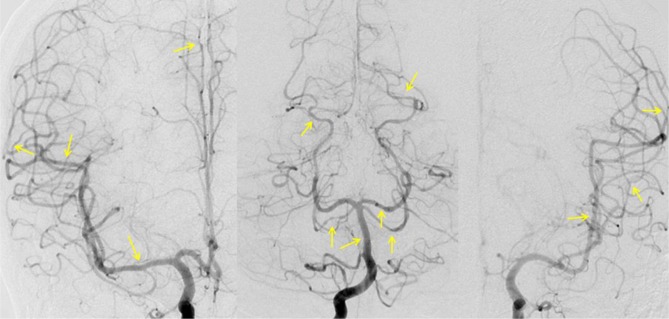
Fig. 5
Complete resolution of vasoconstriction is shown on 6-month follow-up MRA. MRA = MR angiography.
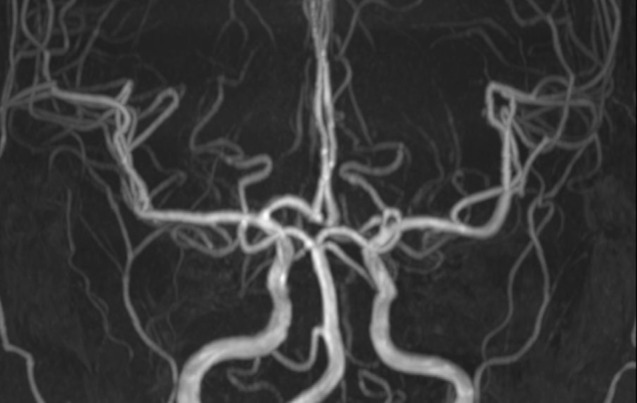
Fig. 6
(A) Brain CT shows deep ICH and associated IVH. Note the symmetrically scattered low density lesions in the bilateral basal ganglia and parietal lobes. (B) There is no vascular abnormality. CT = computed tomography; ICH = intracerebral hemorrhage; IVH = intraventricular hemorrhage.
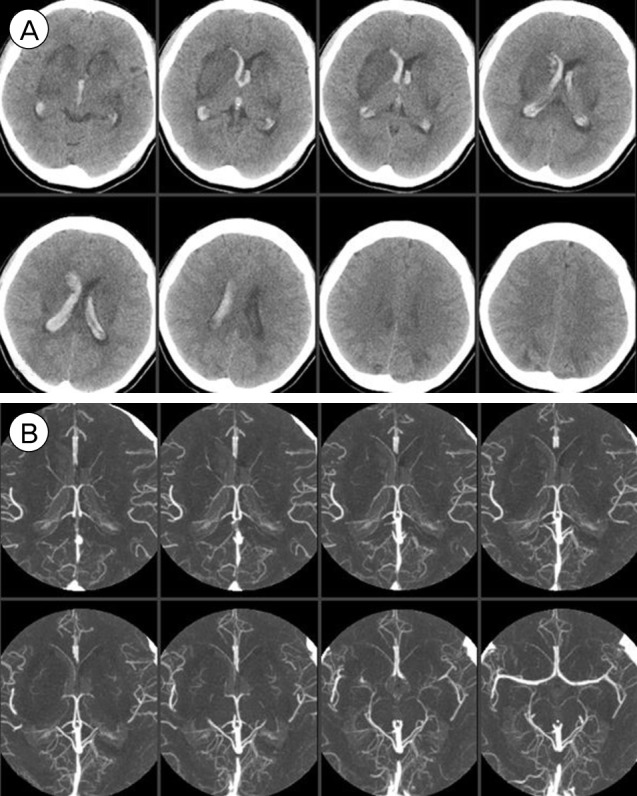
Fig. 7
(A, B) T2 Fluid Attenuated Inversion Recovery (FLAIR) images (A) and apparent diffusion coefficient images (B) suggest vasogenic edema. (C) MRA is normal. MRA = MR angiography.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download